| Virios Therapeutics, Inc. and Wex Pharmaceuticals, Inc. Announce Business Combination to Form Dogwood Therapeutics, Inc. (Nasdaq: “DWTX”) October 7, 2024 The renaming of Virios Therapeutics, Inc. to Dogwood Therapeutics, Inc. is effective on October 9, 2024. The information in this presentation was prepared on the basis that the name change is effected on that date. |
| Forward-Looking Statements ➢ Statements in this presentation contain “forward-looking statements,” within the meaning of the U.S. Private Securities Litigation Reform Act of 1995, that are subject to substantial risks and uncertainties. All statements, other than statements of historical fact, contained in this presentation are forward-looking statements. Forward-looking statements contained in this presentation may be identified by the use of words such as “anticipate,” “believe,” “contemplate,” “could,” “estimate,” “expect,” “intend,” “seek,” “may,” “might,” “plan,” “potential,” “predict,” “project,” “suggest,” “target,” “aim,” “should,” "will,” “would,” or the negative of these words or other similar expressions, although not all forward-looking statements contain these words. Forward-looking statements are based on the current expectations of Dogwood Therapeutics, Inc. (“Dogwood”) and are subject to inherent uncertainties, risks and assumptions that are difficult to predict, including risks related to the completion, timing and results of current and future clinical studies relating to Dogwood’s product candidates. Further, certain forward-looking statements are based on assumptions as to future events that may not prove to be accurate. These and other risks and uncertainties are described more fully in the section titled “Risk Factors” in the Amended Annual Report on Form 10-K/A for the year ended December 31, 2023, filed with the Securities and Exchange Commission. Forward-looking statements contained in this presentation are made as of this date, and Dogwood undertakes no duty to update such information except as required under applicable law. Important Additional Information and Where to Find It ➢ Dogwood, its directors and certain of its executive officers are deemed to be participants in the solicitation of proxies from Dogwood stockholders in connection with Dogwood's expected special meeting seeking stockholder approval of conversion of Dogwood’s preferred stock (“Preferred Stock”)and other matters related to the business combination with Wex Pharmaceuticals, Inc. (the “Combination”.) Information regarding the names of Dogwood’s directors and executive officers and their respective interests in Dogwood by security holdings or otherwise can be found in Virios Therapeutics, Inc.’s proxy statement for its 2024 Annual Meeting of Stockholders, filed with the SEC on April 25, 2024. To the extent holdings of Dogwood’s securities have changed since the amounts set forth in Virios Therapeutics Inc.’s proxy statement for the 2024 Annual Meeting of Stockholders, such changes have been or will be reflected on Initial Statements of Beneficial Ownership on Form 3 or Statements of Change in Ownership on Form 4 filed with the SEC. These documents are available free of charge at the SEC’s website at www.sec.gov. Dogwood intends to file a proxy statement and accompanying proxy card with the SEC in connection with the solicitation of proxies from Dogwood stockholders in connection with Dogwood's expected special meeting seeking stockholder approval of conversion of the Preferred Stock and other matters related to the Combination. Additional information regarding the identity of participants, and their direct or indirect interests, by security holdings or otherwise, will be set forth in Dogwood’s proxy statement for such special meeting, including the schedules and appendices thereto. INVESTORS AND STOCKHOLDERS ARE STRONGLY ENCOURAGED TO READ ANY SUCH PROXY STATEMENT AND THE ACCOMPANYING PROXY CARD AND ANY AMENDMENTS AND SUPPLEMENTS THERETO AS WELL AS ANY OTHER DOCUMENTS FILED BY DOGWOOD WITH THE SEC CAREFULLY AND IN THEIR ENTIRETY WHEN THEY BECOME AVAILABLE AS THEY WILL CONTAIN IMPORTANT INFORMATION. Stockholders will be able to obtain copies of the proxy statement, any amendments or supplements to the proxy statement, the accompanying proxy card, and other documents filed by Dogwood with the SEC for no charge at the SEC’s website at www.sec.gov. Copies will also be available at no charge at the Investor Relations section of Dogwood’s corporate website at https://ir.DWTX.com/ or by contacting Dogwood’s Investor Relations at Dogwood Therapeutics, Inc., 44 Milton Avenue, Alpharetta, GA 30009 or by emailing Dogwood’s Investor Relations at IR@dwtx.com or (866) 620-8655. 2 Forward-Looking Statements and Disclaimers |
| Virios Therapeutics, Inc. and Wex Pharmaceuticals, Inc. Announce Business Combination to Form Dogwood Therapeutics, Inc. (Nasdaq: “DWTX”) ➢ Expanded pipeline includes a potential first-in-class non-opioid, NaV1.7 inhibition pain treatment, Halneuron® , currently in Phase 2b development for chemotherapy-induced neuropathic pain (CINP) ➢ Strategic financing by CK Life-Sciences Int’l., (Holdings) Inc., and existing cash provides working capital of approximately $23 million to fund operations to Q4 2025 ❖ Enables Halneuron® Phase 2b development to interim data readout 2H 2025 ➢ IMC-2 Long-COVID Phase 2a study expected in early Q4 2024 ➢ Existing VIRI stockholders to be granted a contingent value right (“CVR”) tied to potential milestone payments associated with any future corporate partnering transaction for IMC-1 and IMC-2, effective October 17th , 2024 ➢ Company announces 25-for-1 reverse stock split of its common stock to facilitate the new business combination and restore Nasdaq listing compliance, effective October 9 th ➢ Combined team has extensive experience in developing and/or commercializing pain medicines, including Celebrex, Lyrica and Savella 3 |
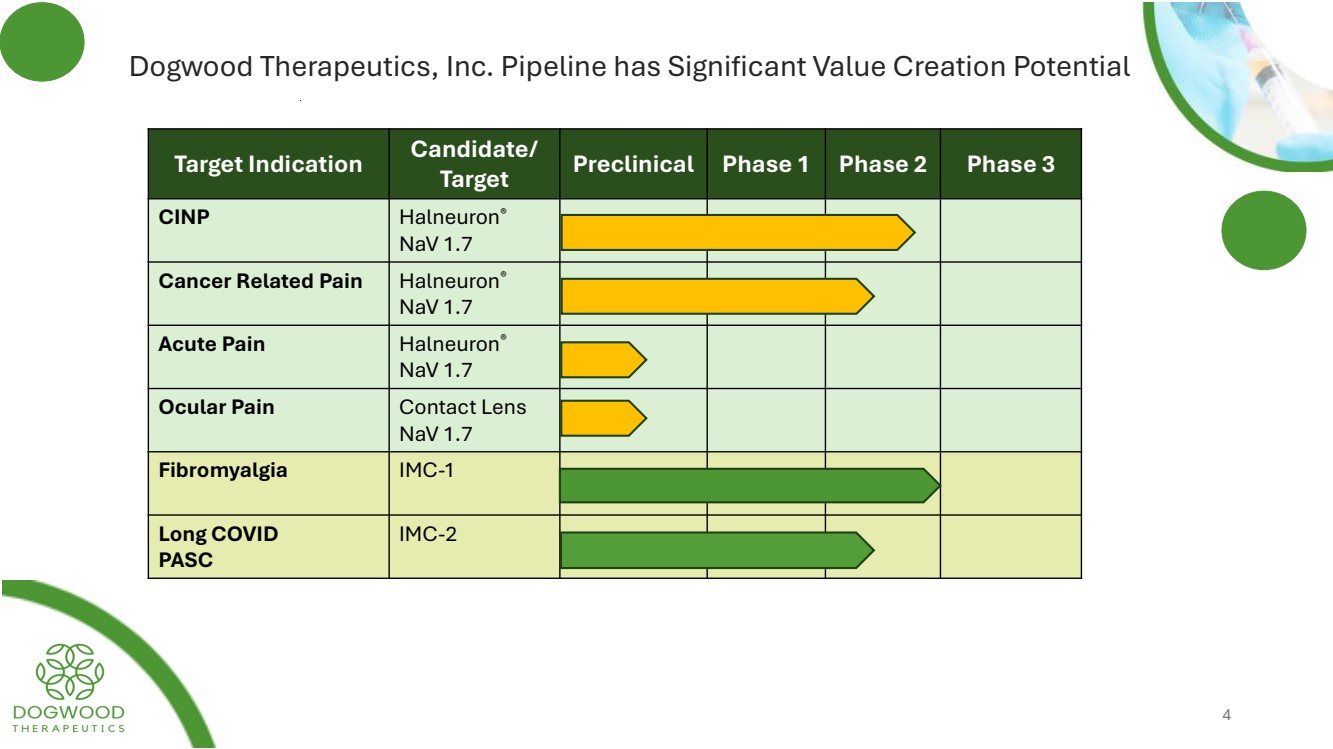
| Dogwood Therapeutics, Inc. Pipeline has Significant Value Creation Potential Target Indication Candidate/ Target Preclinical Phase 1 Phase 2 Phase 3 CINP Halneuron® NaV 1.7 Cancer Related Pain Halneuron® NaV 1.7 Acute Pain Halneuron® NaV 1.7 Ocular Pain Contact Lens NaV 1.7 Fibromyalgia IMC-1 Long COVID PASC IMC-2 4 |
| WEX Pharmaceuticals Inc. Overview ➢ WEX Pharmaceuticals (“WEX”), headquartered in Canada, is a pharmaceutical company focused on the development of Halneuron® , a non-opioid pain medication to treat moderate to severe pain ➢ WEX was a wholly owned subsidiary of CK Life Sciences Int'l., (Holdings) Inc., a publicly listed company in Hong Kong (SEHK:0775) ➢ Key asset Halneuron® , which has been granted Fast Track Designation from the FDA as a treatment for Halneuron® CINP, has been previously tested in over 700 patients 5 |
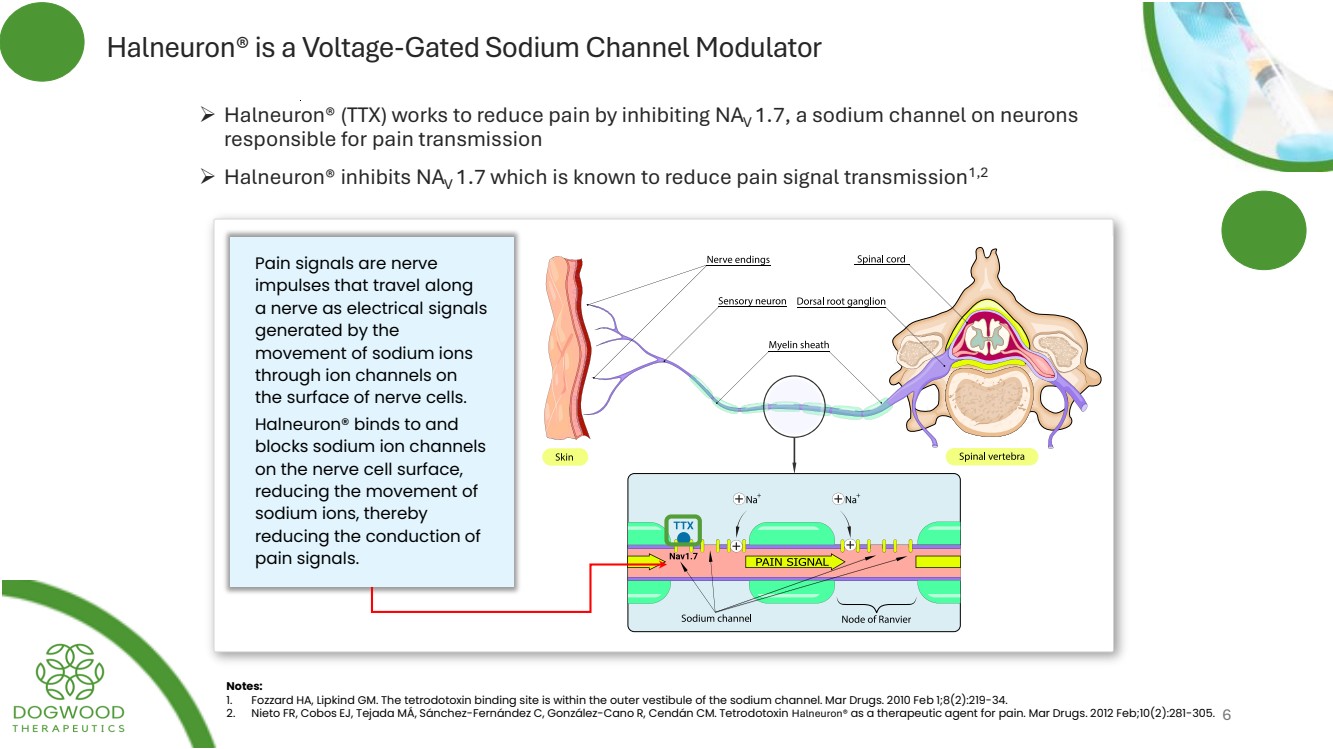
| Halneuron® is a Voltage-Gated Sodium Channel Modulator ➢ Halneuron® (TTX) works to reduce pain by inhibiting NAV 1.7, a sodium channel on neurons responsible for pain transmission ➢ Halneuron® inhibits NAV 1.7 which is known to reduce pain signal transmission1,2 Pain signals are nerve impulses that travel along a nerve as electrical signals generated by the movement of sodium ions through ion channels on the surface of nerve cells. Halneuron® binds to and blocks sodium ion channels on the nerve cell surface, reducing the movement of sodium ions, thereby reducing the conduction of pain signals. Notes: 1. Fozzard HA, Lipkind GM. The tetrodotoxin binding site is within the outer vestibule of the sodium channel. Mar Drugs. 2010 Feb 1;8(2):219-34. 2. Nieto FR, Cobos EJ, Tejada MÁ, Sánchez-Fernández C, González-Cano R, Cendán CM. Tetrodotoxin Halneuron® as a therapeutic agent for pain. Mar Drugs. 2012 Feb;10(2):281-305. 6 |
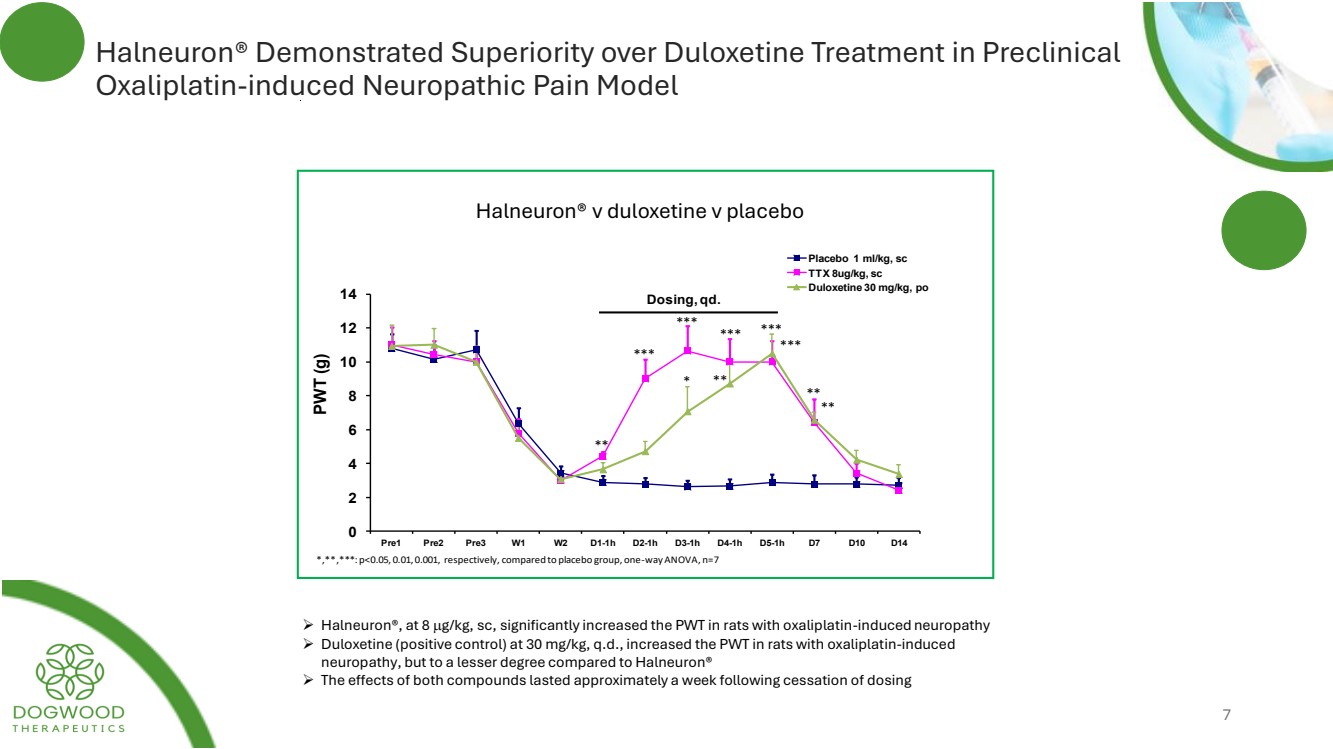
| Halneuron® Demonstrated Superiority over Duloxetine Treatment in Preclinical Oxaliplatin-induced Neuropathic Pain Model ➢ Halneuron®, at 8 g/kg, sc, significantly increased the PWT in rats with oxaliplatin-induced neuropathy ➢ Duloxetine (positive control) at 30 mg/kg, q.d., increased the PWT in rats with oxaliplatin-induced neuropathy, but to a lesser degree compared to Halneuron® ➢ The effects of both compounds lasted approximately a week following cessation of dosing 7 0 2 4 6 8 10 12 14 Pre1 Pre2 Pre3 W1 W2 D1-1h D2-1h D3-1h D4-1h D5-1h D7 D10 D14 PWT (g) Effect of TTX on 1h PWT in Oxaliplatin induced pain model rats Placebo 1 ml/kg, sc TTX 8ug/kg, sc Duloxetine 30 mg/kg, po Dosing, qd. * ** *** ** *** ** ** *,**,***: p<0.05, 0.01, 0.001, respectively, compared to placebo group, one-way ANOVA, n=7 *** *** *** Halneuron® v duloxetine v placebo |
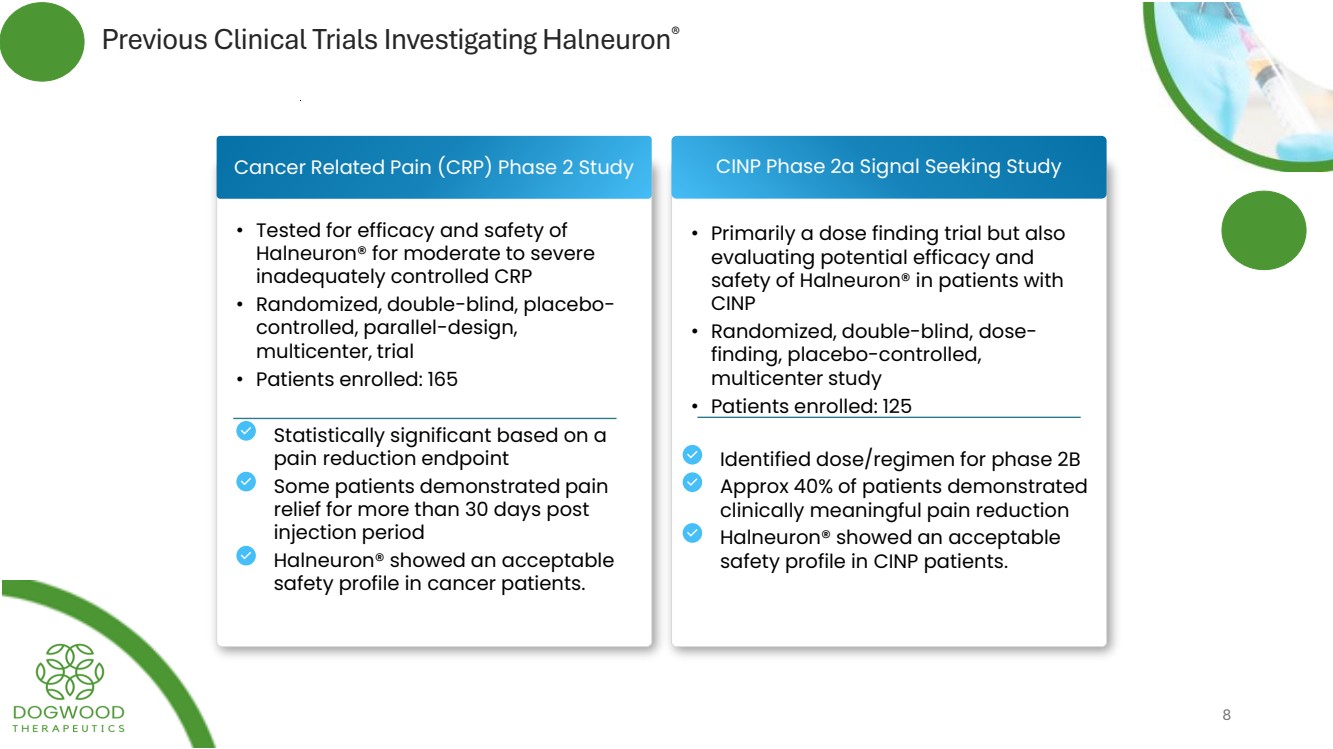
| Previous Clinical Trials Investigating Halneuron® CINP Phase 2a Signal Seeking Study • Tested for efficacy and safety of Halneuron® for moderate to severe inadequately controlled CRP • Randomized, double-blind, placebo-controlled, parallel-design, multicenter, trial • Patients enrolled: 165 • Primarily a dose finding trial but also evaluating potential efficacy and safety of Halneuron® in patients with CINP • Randomized, double-blind, dose-finding, placebo-controlled, multicenter study • Patients enrolled: 125 Cancer Related Pain (CRP) Phase 2 Study Statistically significant based on a pain reduction endpoint Some patients demonstrated pain relief for more than 30 days post injection period Halneuron® showed an acceptable safety profile in cancer patients. Identified dose/regimen for phase 2B Approx 40% of patients demonstrated clinically meaningful pain reduction Halneuron® showed an acceptable safety profile in CINP patients. 8 |
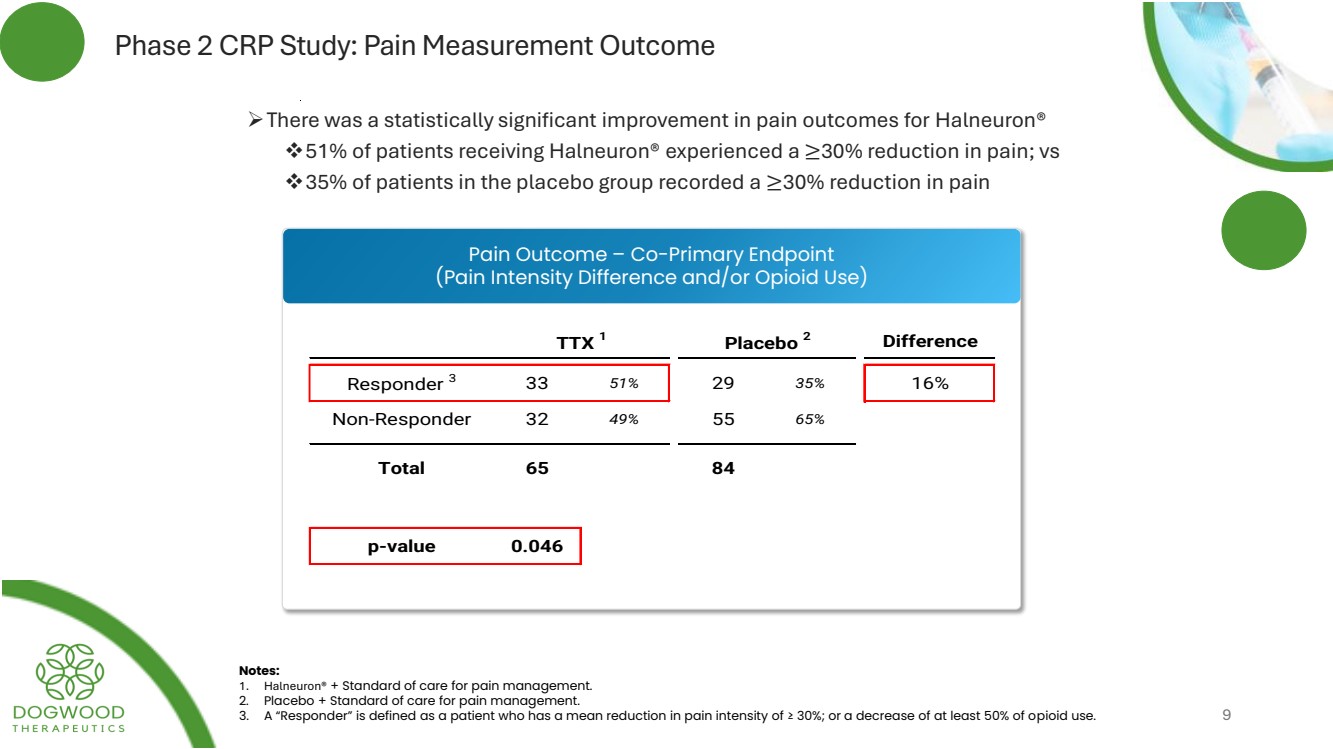
| Phase 2 CRP Study: Pain Measurement Outcome ➢There was a statistically significant improvement in pain outcomes for Halneuron® ❖51% of patients receiving Halneuron® experienced a ≥30% reduction in pain; vs ❖35% of patients in the placebo group recorded a ≥30% reduction in pain Notes: 1. Halneuron® + Standard of care for pain management. 2. Placebo + Standard of care for pain management. 3. A “Responder” is defined as a patient who has a mean reduction in pain intensity of ≥ 30%; or a decrease of at least 50% of opioid use. 9 Pain Outcome – Co-Primary Endpoint (Pain Intensity Difference and/or Opioid Use) TTX 1 Placebo 2 Difference Responder 3 33 51% 29 35% 16% Non-Responder 32 49% 55 65% Total 65 84 95% C. I. 0.4 - 32.1 p-value 0.046 |
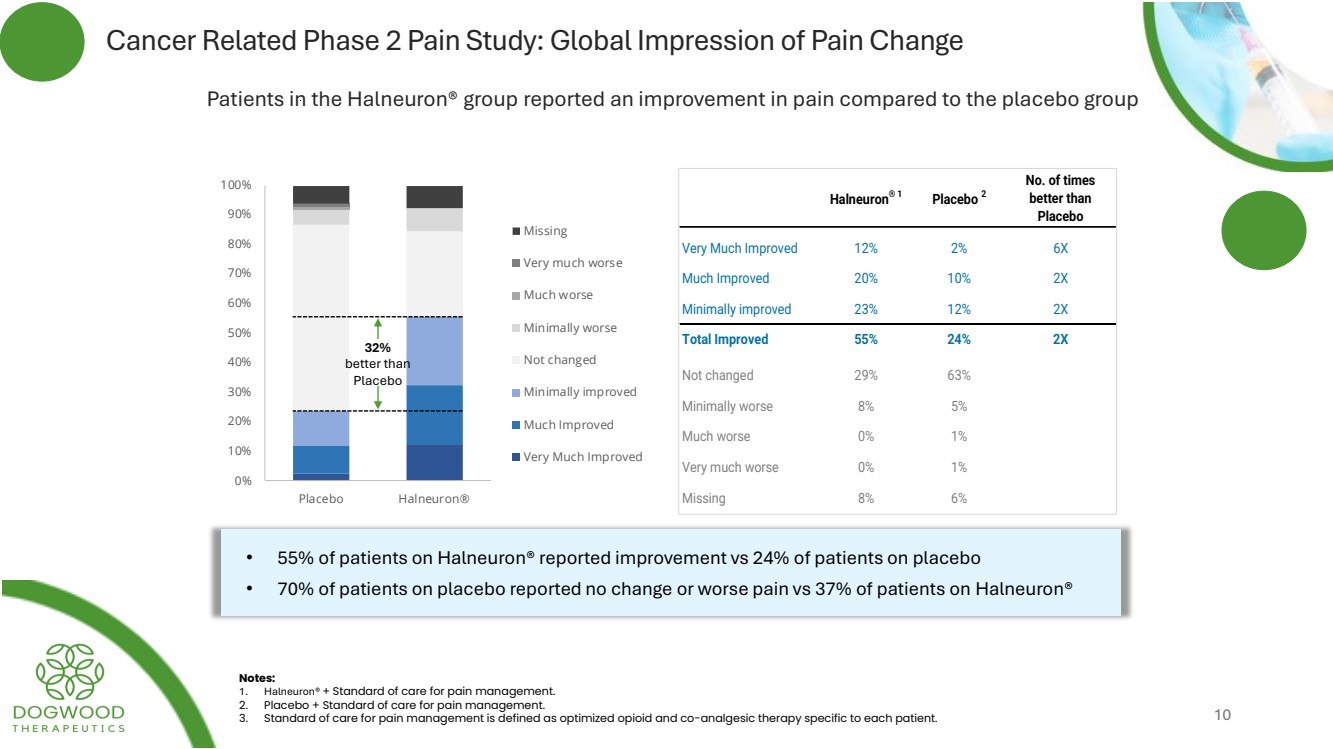
| Cancer Related Phase 2 Pain Study: Global Impression of Pain Change Patients in the Halneuron® group reported an improvement in pain compared to the placebo group 0 % 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% Placebo Halneuron® Missing Very much worse Much worse Minimally worse Not changed Minimally improved Much Improved Very Much Improved 32% better than Placebo • 55% of patients on Halneuron® reported improvement vs 24% of patients on placebo • 70% of patients on placebo reported no change or worse pain vs 37% of patients on Halneuron® Notes: 1. Halneuron® + Standard of care for pain management. 2. Placebo + Standard of care for pain management. 3. Standard of care for pain management is defined as optimized opioid and co-analgesic therapy specific to each patient. Halneuron® 1 Placebo 2 No. of times better than Placebo Very Much Improved 12% 2% 6X Much Improved 20% 10% 2X Minimally improved 23% 12% 2X Total Improved 55% 24% 2X Not changed 29% 63% Minimally worse 8% 5% Much worse 0% 1% Very much worse 0% 1% Missing 8% 6% 10 |
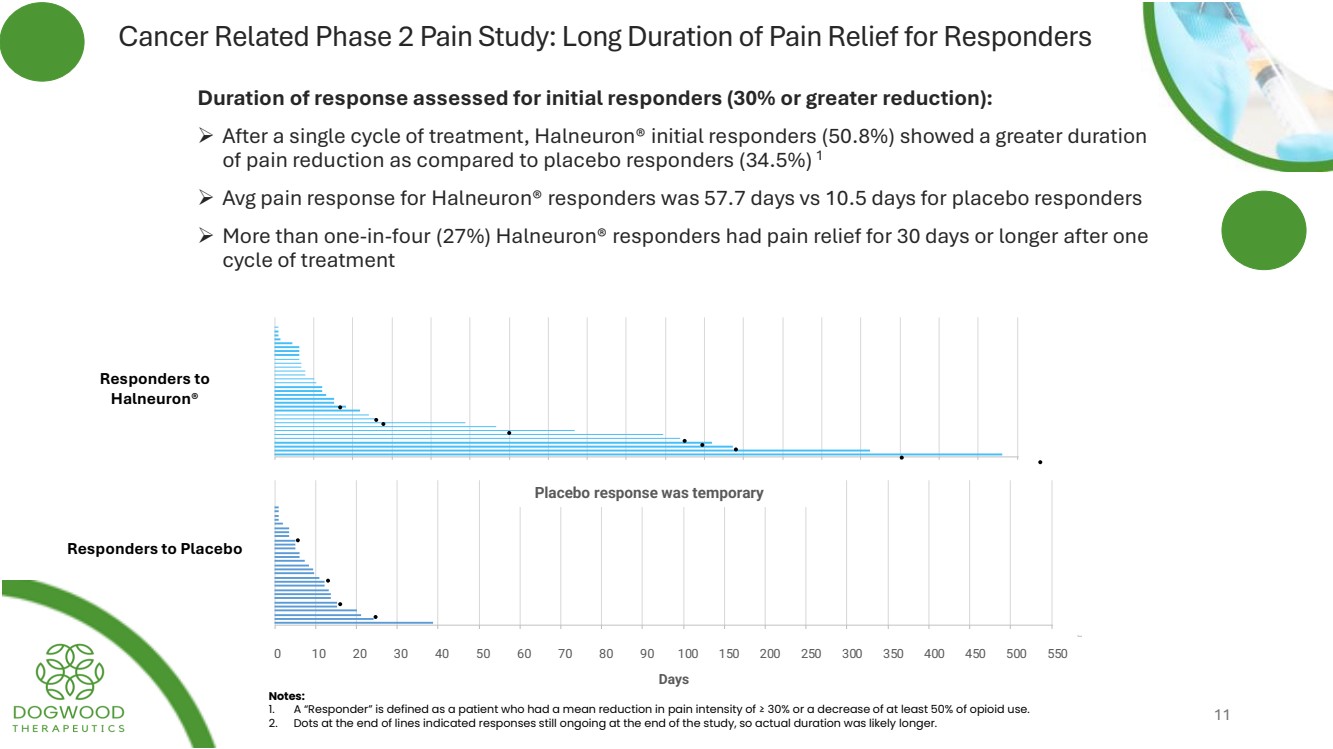
| Cancer Related Phase 2 Pain Study: Long Duration of Pain Relief for Responders Duration of response assessed for initial responders (30% or greater reduction): ➢ After a single cycle of treatment, Halneuron® initial responders (50.8%) showed a greater duration of pain reduction as compared to placebo responders (34.5%) 1 ➢ Avg pain response for Halneuron® responders was 57.7 days vs 10.5 days for placebo responders ➢ More than one-in-four (27%) Halneuron® responders had pain relief for 30 days or longer after one cycle of treatment Placebo response was temporary Notes: 1. A “Responder” is defined as a patient who had a mean reduction in pain intensity of ≥ 30% or a decrease of at least 50% of opioid use. 2. Dots at the end of lines indicated responses still ongoing at the end of the study, so actual duration was likely longer. Responders to Halneuron® Responders to Placebo 0 10 20 30 40 50 60 70 80 90 100 150 200 250 300 350 400 450 500 550 Days 11 |
| Halneuron Five-arm Study, Assessing Three Doses and Two Dose Regimens: ➢ Halneuron® high doses delivered greater pain reduction as compared to low doses ➢ Halneuron® QD dose pain reduction comparable to BID dose, but exhibited better tolerability ➢ Halneuron® pain relief evident four weeks post treatment ➢ Halneuron® high doses delivered clinically meaningful pain reduction for 35-40% of patients 12 CINP 4-week Phase 2a Signal Seeking Study: Trial Conclusions |
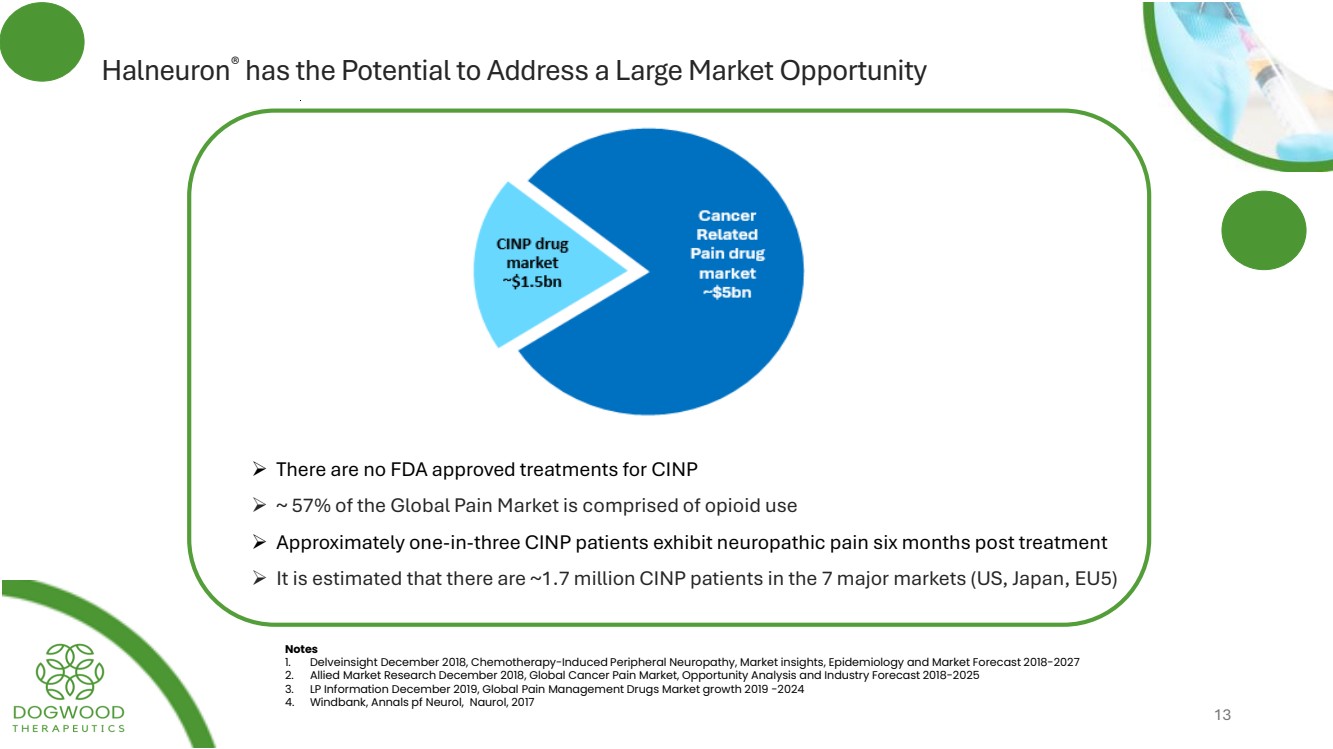
| Halneuron® has the Potential to Address a Large Market Opportunity Notes 1. Delveinsight December 2018, Chemotherapy-Induced Peripheral Neuropathy, Market insights, Epidemiology and Market Forecast 2018-2027 2. Allied Market Research December 2018, Global Cancer Pain Market, Opportunity Analysis and Industry Forecast 2018-2025 3. LP Information December 2019, Global Pain Management Drugs Market growth 2019 -2024 4. Windbank, Annals pf Neurol, Naurol, 2017 ➢ There are no FDA approved treatments for CINP ➢ ~ 57% of the Global Pain Market is comprised of opioid use ➢ Approximately one-in-three CINP patients exhibit neuropathic pain six months post treatment ➢ It is estimated that there are ~1.7 million CINP patients in the 7 major markets (US, Japan, EU5) 13 |
| Halneuron® - A Novel Non-opioid Pain Development Candidate Lead indication: CINP Represents a Large Market Opportunity o Currently there are no treatments approved for Chemotherapy-Induced Neuropathic Pain Validated Mechanism: Pain blocking process well-validated by decades of scientific research o Halneuron® preclinical data support potential to reduce pain Reduced Pain in Both CRP and CINP Human Clinical Trials o Halneuron® Exhibits Acceptable Safety Profile Experienced Team: Combined team has track record of developing and/or commercializing blockbuster medicines, including pain therapeutics (e.g. Celebrex, Lyrica and Savella) Notes: 1. Delveinsight December 2018, Chemotherapy-Induced Peripheral Neuropathy, Market insights, Epidemiology and Market Forecast 2018-2027 2. Allied Market Research December 2018, Global Cancer Pain Market, Opportunity Analysis and Industry Forecast 2018-2025 14 Novel Nav 1.7 voltage-gated sodium channel inhibitor therapeutic o Highly differentiated, non-opioid mechanism of action to treat pain |
| Novel Combination Antiviral Program 15 ➢ Two novel, late-stage clinical stage development assets: ➢ IMC-1 (famciclovir + celecoxib) ready for Phase 3 development as treatment for FM: ➢ Phase 2a and Phase 2b in Fibromyalgia (“FM”) ➢ FDA agreement to enter Phase 3 post EoP2 meeting ➢ Exploring Phase 3 partnership and extended-release dosage formulation to extend IP ➢ IMC-2 (valacyclovir + celecoxib) Phase 2 Long-COVID study ongoing: ➢ Proof of concept completed in study 2023, new IP filed with protection potential to 2044 ➢ We have clarity from FDA on the development requirements associated with advancing IMC-2 into Phase 2 development as a treatment for Long-COVID symptoms ➢ Three-arm, Phase 2 investigator-initiated study of IMC-2 enrolled at Bateman-Horne Center, topline data expected in early Q4 2024 |
| IMC-1 Phase 3 Study Designs Agreed To By FDA I. Pharmacokinetic/Food Effect Study II. Study 1: Head-to-Head IMC-1 vs Placebo (n=320) ❖ 1:1 Randomization – 160 in each group ❖ Primary Endpoint – Reduction in Pain at 12 Weeks III. Study 2: Multifactorial Study of IMC-1 vs Placebo vs Famciclovir vs Celecoxib (n=640) ❖ 1:1:1:1 Randomization – 160 each group ❖ Primary Endpoint – Reduction in Pain at 12 Weeks IV. Study 3: Long-term safety extension study ❖ Treatment with IMC-1 for a year (n = 300 subjects at 6 months and 100 subjects at 1 year) 16 |
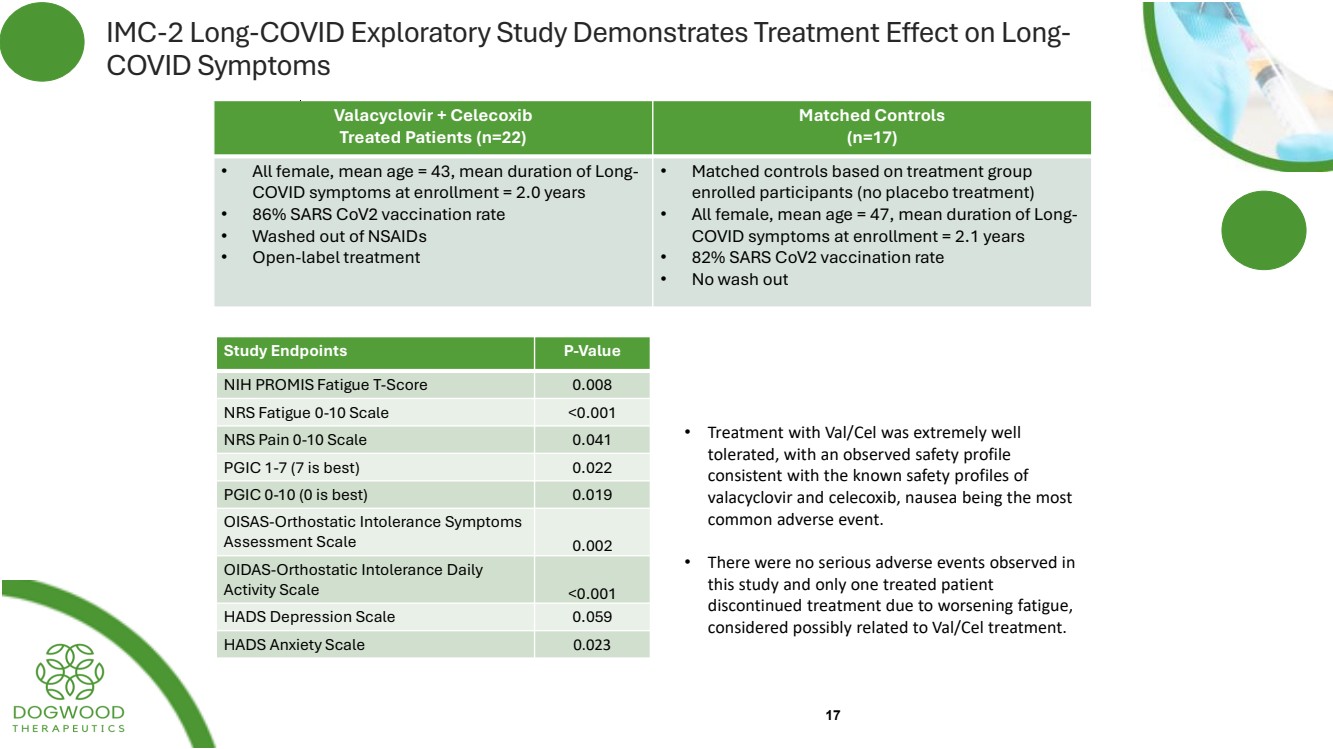
| IMC-2 Long-COVID Exploratory Study Demonstrates Treatment Effect on Long-COVID Symptoms 17 Valacyclovir + Celecoxib Treated Patients (n=22) Matched Controls (n=17) • All female, mean age = 43, mean duration of Long-COVID symptoms at enrollment = 2.0 years • 86% SARS CoV2 vaccination rate • Washed out of NSAIDs • Open-label treatment • Matched controls based on treatment group enrolled participants (no placebo treatment) • All female, mean age = 47, mean duration of Long-COVID symptoms at enrollment = 2.1 years • 82% SARS CoV2 vaccination rate • No wash out Study Endpoints P-Value NIH PROMIS Fatigue T-Score 0.008 NRS Fatigue 0-10 Scale <0.001 NRS Pain 0-10 Scale 0.041 PGIC 1-7 (7 is best) 0.022 PGIC 0-10 (0 is best) 0.019 OISAS-Orthostatic Intolerance Symptoms Assessment Scale 0.002 OIDAS-Orthostatic Intolerance Daily Activity Scale <0.001 HADS Depression Scale 0.059 HADS Anxiety Scale 0.023 • Treatment with Val/Cel was extremely well tolerated, with an observed safety profile consistent with the known safety profiles of valacyclovir and celecoxib, nausea being the most common adverse event. • There were no serious adverse events observed in this study and only one treated patient discontinued treatment due to worsening fatigue, considered possibly related to Val/Cel treatment. |
| Bateman Horne Center Follow-Up 202 PASC Study ➢ Study also run by Bateman Horne Center, Salt Lake City, Utah ➢ Second IRB approved study supported by Virios via unrestricted, investigator-initiated grant ➢ Dr. Lucinda Bateman, MD, a recognized leader in both Long-COVID and fatigue related clinical research, serves as BHC 202 primary investigator ➢ Enrollment commenced in December 2023: 3 Arms 1:1:1 randomization, double blinded and randomized study: ➢ Dosing: Val/Cel BID, two doses v. Placebo BID ➢ Primary Endpoint: fatigue reduction ➢ Secondary Endpoints assessments: sleep, orthostatic symptoms, anxiety, depression and overall health ➢ Top line results: Early Q4 2024 18 |
| Virios Therapeutics, Inc. and Wex Pharmaceuticals, Inc. Announce Business Combination to Form Dogwood Therapeutics, Inc. (Nasdaq: “DWTX”) ➢ Expanded pipeline includes a potential first-in-class non-opioid, NaV1.7 inhibition pain treatment, Halneuron® , currently in Phase 2b development for chemotherapy-induced neuropathic pain (CINP) ➢ Strategic financing by CK Life-Sciences Int’l., (Holdings) Inc., and existing cash provides working capital of approximately $23 million to fund operations to Q4 2025 ❖ Enables Halneuron® Phase 2b development to interim data readout 2H 2025 ➢ IMC-2 Long-COVID Phase 2a study expected in early Q4 2024 ➢ Existing VIRI stockholders to be granted a contingent value right (“CVR”) tied to potential milestone payments associated with any future corporate partnering transaction for IMC-1 and IMC-2, effective October 17th , 2024 ➢ Company announces 25-for-1 reverse stock split of its common stock to facilitate the new business combination and restore Nasdaq listing compliance, effective October 9 th ➢ Combined team has extensive experience in developing and/or commercializing pain medicines, including Celebrex, Lyrica and Savella 19 |