| HSV - 1 virus FORTRESS STUDY SUMMARY Topline Results September 19, 2022 1 |
| Forward Looking Statements 2 • Statements in this presentation contain “forward - looking statements” that are subject to substantial risks and uncertainties. Fo rward - looking statements contained in this presentation may be identified by the use of words such as “anticipate,” “expect,” “believe,” “will,” “may,” “should,” “estimate,” “project,” “ou tlo ok,” “forecast” or other similar words, and include, without limitation, all statements other than those regarding historical facts, statements regarding Virios Therapeutics, Inc.’s expectations regardi ng our future financial or business performance, plans, prospects, trends or strategies, objectives of management, competition and other financial and business matters; the potential, safety, eff icacy, and regulatory and clinical progress of our current and prospective product candidates, planned clinical trials and preclinical activities, and projected research and development costs ; the estimated size of the market for our product candidates; and the timing and success of our development and commercialization of our anticipated product candidates and the market acceptance thereof. Forward - looking statements are based on our current expectations and are subject to inherent uncertainties, risks and assumptions that are difficult to predict. Further, ce rtain forward - looking statements are based on assumptions as to future events that may not prove to be accurate. These statements are neither promises nor guarantees, but involve known and unk nown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or ach ievements expressed or implied by the forward - looking statements, including, but not limited to, the following: the ongoing effects of COVID - 19 has adversely impacted and may continue to adversely impact our business, including our preclinical studies and clinical trials; our limited operating history, which may make it difficult to evaluate our current business and pre dict our future success and viability; we have and expect to continue to incur significant losses; our need for additional funding, which may not be available; our substantial dependence on the succ ess of our lead product candidates; failure to identify additional product candidates and develop or commercialize marketable products; the early stage of our development efforts; potential un for eseen events during clinical trials could cause delays or other adverse consequences; risks relating to the regulatory approval process or ongoing regulatory obligations; our product candid ate s may cause serious adverse side effects; our reliance on third parties; effects of significant competition; the possibility of system failures or security breaches; risks relating to intel lec tual property; our ability to attract, retain and motivate qualified personnel; and significant costs as a result of operating as a public company. These and other risks and uncertainties are de scr ibed more fully in the section titled “Risk Factors” in the Annual Report on Form 10 - K for the year ended December 31, 2021 filed with the Securities and Exchange Commission (“SEC”) and elsewhere in our filings and reports with the SEC. While we may elect to update these forward - looking statements at some point in the future, we assume no obligation to update or revise any fo rward - looking statements except to the extent required by applicable law. Although we believe the expectations reflected in such forward - looking statements are reasonable, we can give no assurance that such expectations will prove to be correct. Accordingly, readers are cautioned not to place undue reliance on these forward - looking statements. No representations or warran ties (expressed or implied) are made about the accuracy of any such forward - looking statements. • This presentation also contains estimates and other statistical data made by independent parties and by us relating to market si ze and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such data and estimates. In addition, p rojections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncerta int y and risk. Neither we nor our affiliates, advisors or representatives makes any representation as to the accuracy or completeness of that data or undertake to update such data aft er the date of this presentation. • You should read the documents that we have filed with the SEC for more complete information about us. We encourage you to rea d s uch documents in full for more detailed information on statistics, reports and clinical trials referenced in this presentation. You may access these documents for free by visiting ED GAR on the SEC website at http://www.sec.gov. |
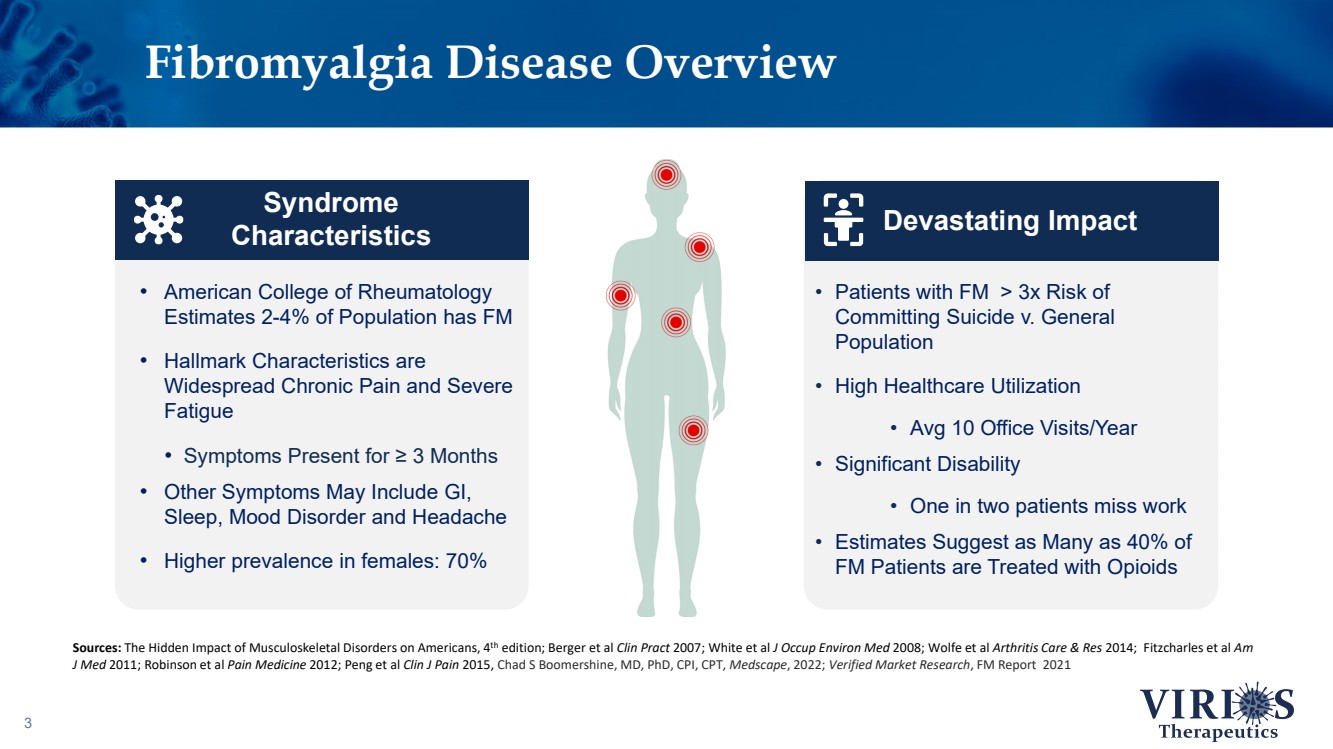
| Fibromyalgia Disease Overview 3 • American College of Rheumatology Estimates 2 - 4% of Population has FM • Hallmark Characteristics are Widespread Chronic Pain and Severe Fatigue • Symptoms Present for ≥ 3 Months • Other Symptoms May Include GI, Sleep, Mood Disorder and Headache • Higher prevalence in females: 70% Syndrome Characteristics Sources: The Hidden Impact of Musculoskeletal Disorders on Americans, 4 th edition; Berger et al Clin Pract 2007; White et al J Occup Environ Med 2008; Wolfe et al Arthritis Care & Res 2014; Fitzcharles et al Am J Med 2011; Robinson et al Pain Medicine 2012; Peng et al Clin J Pain 2015, Chad S Boomershine , MD, PhD, CPI, CPT, Medscape , 2022; Verified Market Research , FM Report 2021 • Patients with FM > 3x Risk of Committing Suicide v. General Population • High Healthcare Utilization • Avg 10 Office Visits/Year • Significant Disability • One in two patients miss work • Estimates Suggest as Many as 40% of FM Patients are Treated with Opioids Devastating Impact |
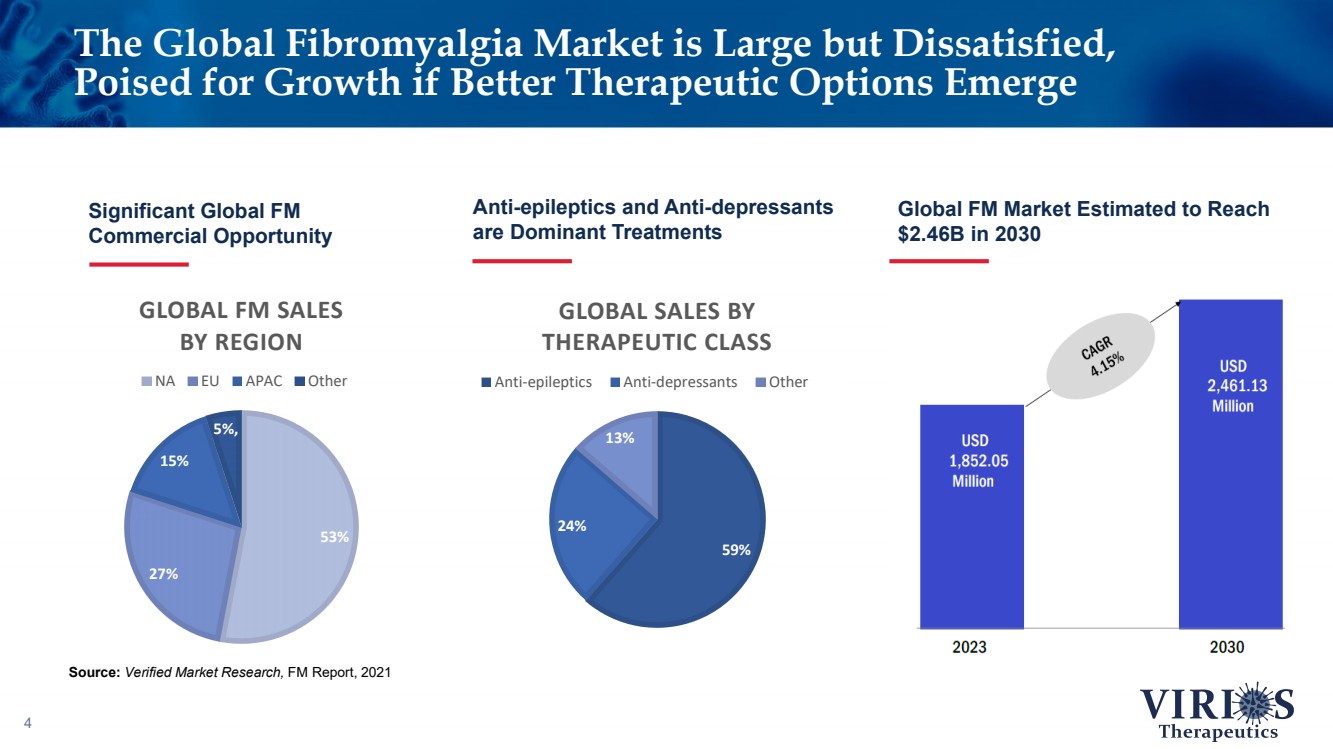
| The Global Fibromyalgia Market is Large but Dissatisfied, Poised for Growth if Better Therapeutic Options Emerge 4 Significant Global FM Commercial Opportunity Source: Verified Market Research, FM Report, 2021 Anti - epileptics and Anti - depressants are Dominant Treatments Global FM Market Estimated to Reach $2.46B in 2030 75% 25% 59% 24% 13% GLOBAL SALES BY THERAPEUTIC CLASS Anti-epileptics Anti-depressants Other 53% 27% 15% 5% , GLOBAL FM SALES BY REGION NA EU APAC Other |
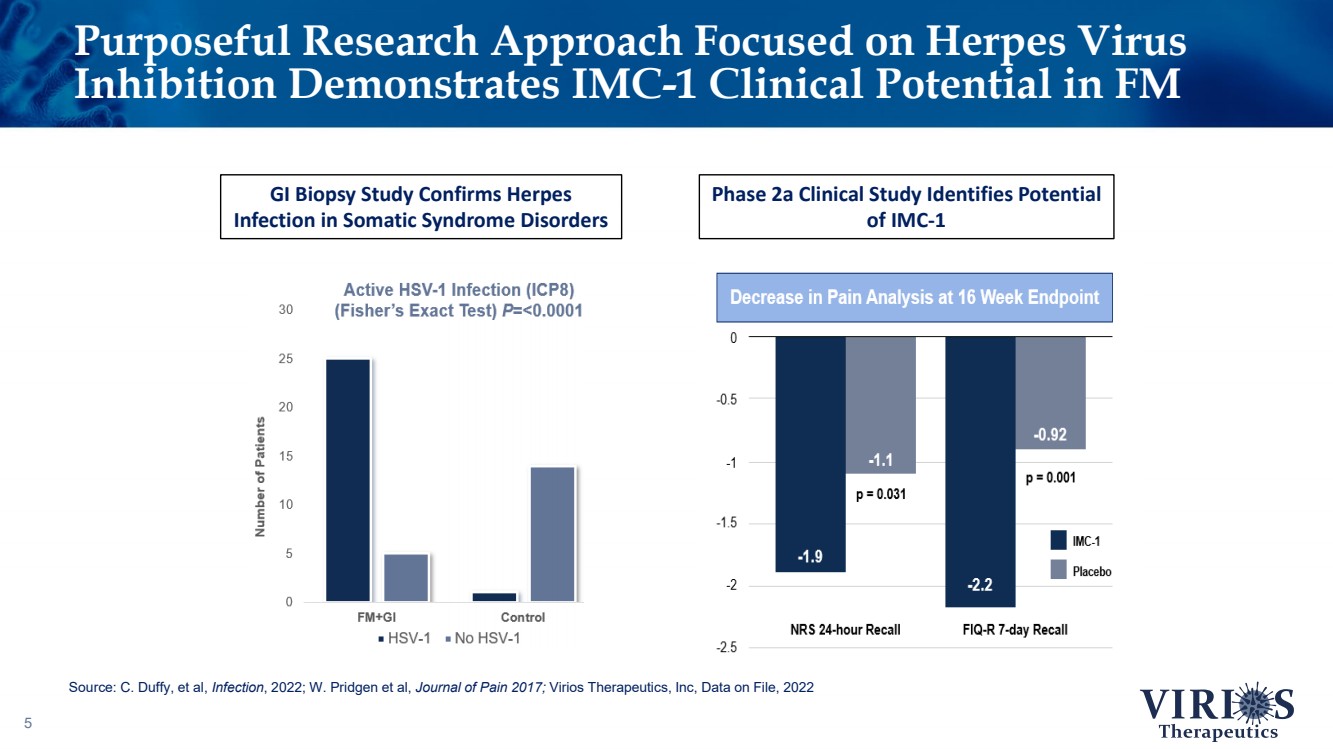
| Purposeful Research Approach Focused on Herpes Virus Inhibition Demonstrates IMC - 1 Clinical Potential in FM 5 GI Biopsy Study Confirms Herpes Infection in Somatic Syndrome Disorders Phase 2a Clinical Study Identifies Potential of IMC - 1 Source: C. Duffy, et al, Infection , 2022; W. Pridgen et al, Journal of Pain 2017; Virios Therapeutics, Inc, Data on File, 2022 |
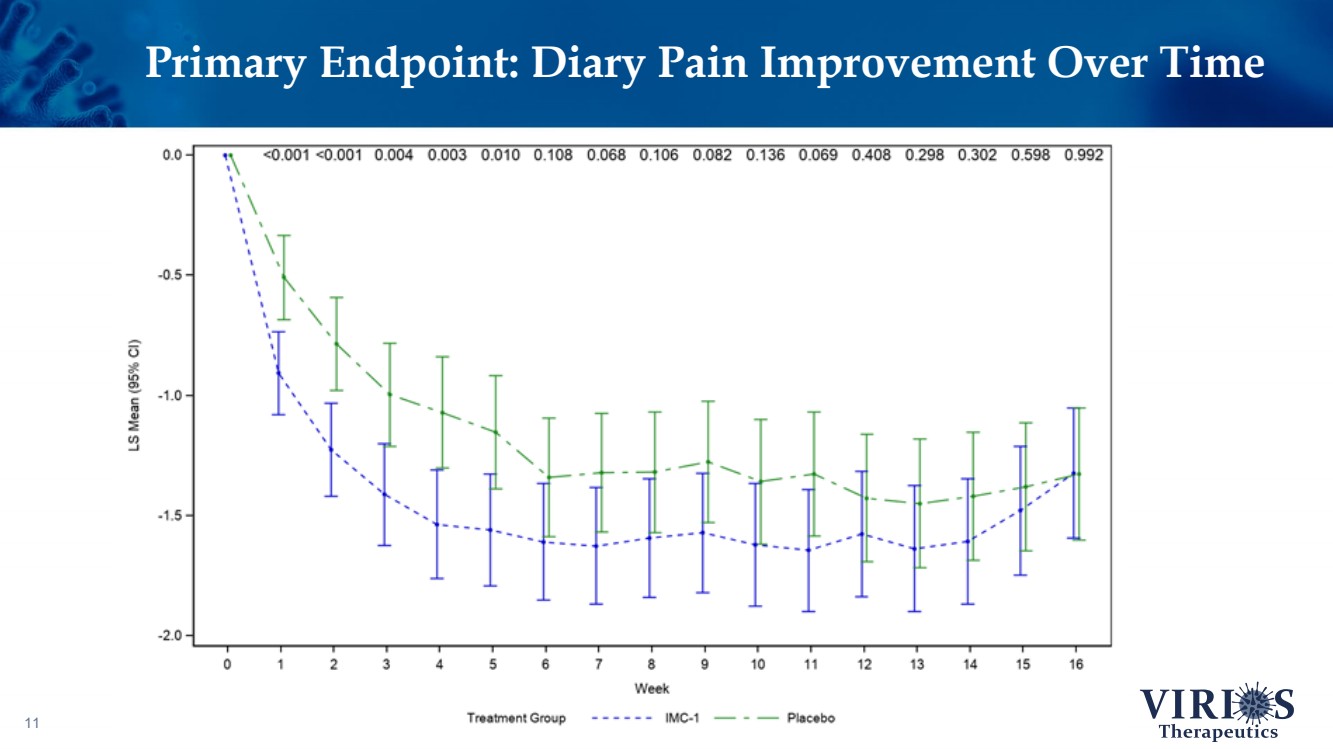
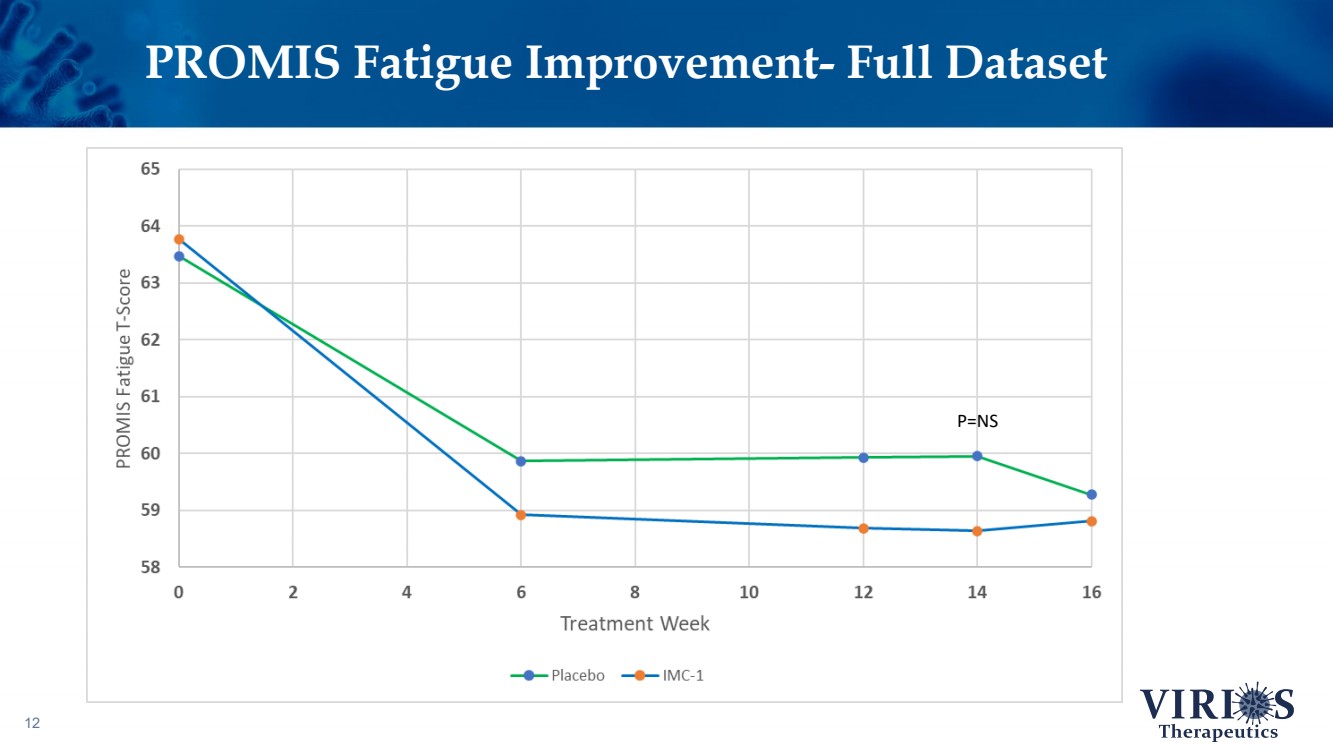
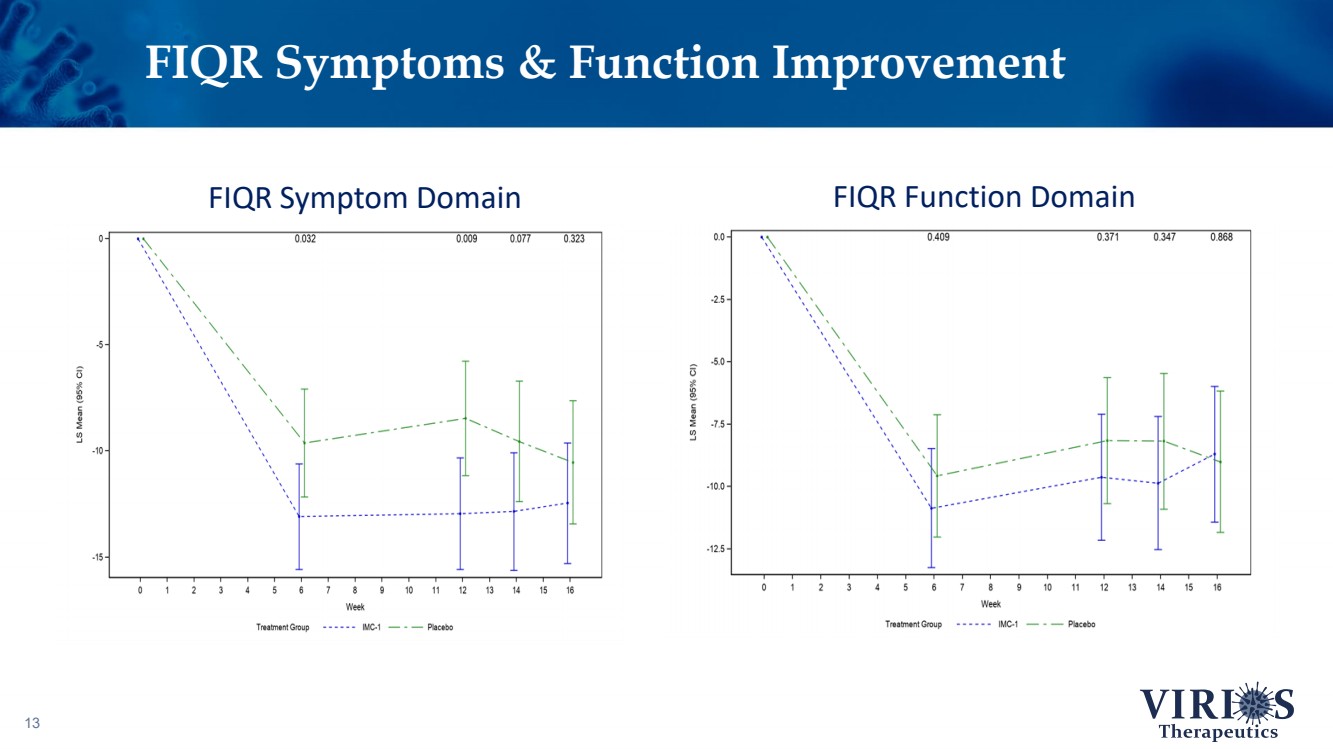
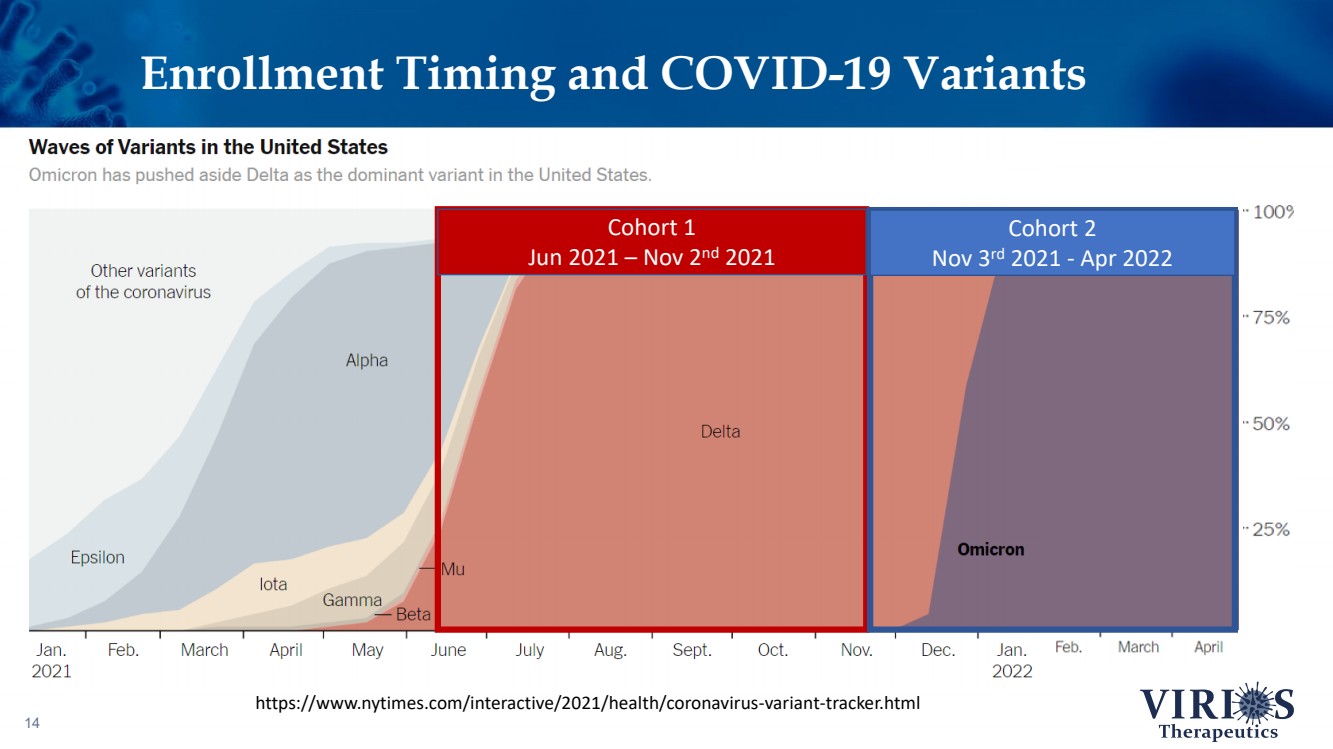
| FORTRESS Study Highlights 6 ❖ Overall, IMC - 1 did not achieve statistical significance on the primary efficacy endpoint of change from baseline to Week 14 in the weekly average of daily self - reported pain scores comparing IMC - 1 and placebo (p=0.302) ❖ Bifurcation of response based on the timing of patient enrollment in the trial: – Cohort 1 Enrollment (June 2021 - Nov 2 nd 2021, n=208): Delta variant of COVID - 19 was the dominant strain in the US, IMC - 1 demonstrated no significant improvement versus placebo treated patients – Cohort 2 Enrollment (Nov 3 rd 2021 - Apr 15 th 2022, n=214): Omicron variant of COVID - 19 dominant, IMC - 1 treated patients demonstrated statistically significant improvement on key outcome measures including: • The primary pain reduction endpoint (p=0.030) • Secondary outcome of PROMIS fatigue (p=0.006) • Key secondary Fibromyalgia Impact Questionnaire - Revised (FIQR) symptoms domain (p=0.015) ❖ IMC - 1 was very well tolerated – Discontinuation due to adverse events occurred in only 4.6% of IMC - 1 treated patients, as compared to 8.1% of placebo treated patients. ❖ Team is presently exploring best opportunities for development of IMC - 1 going forward |
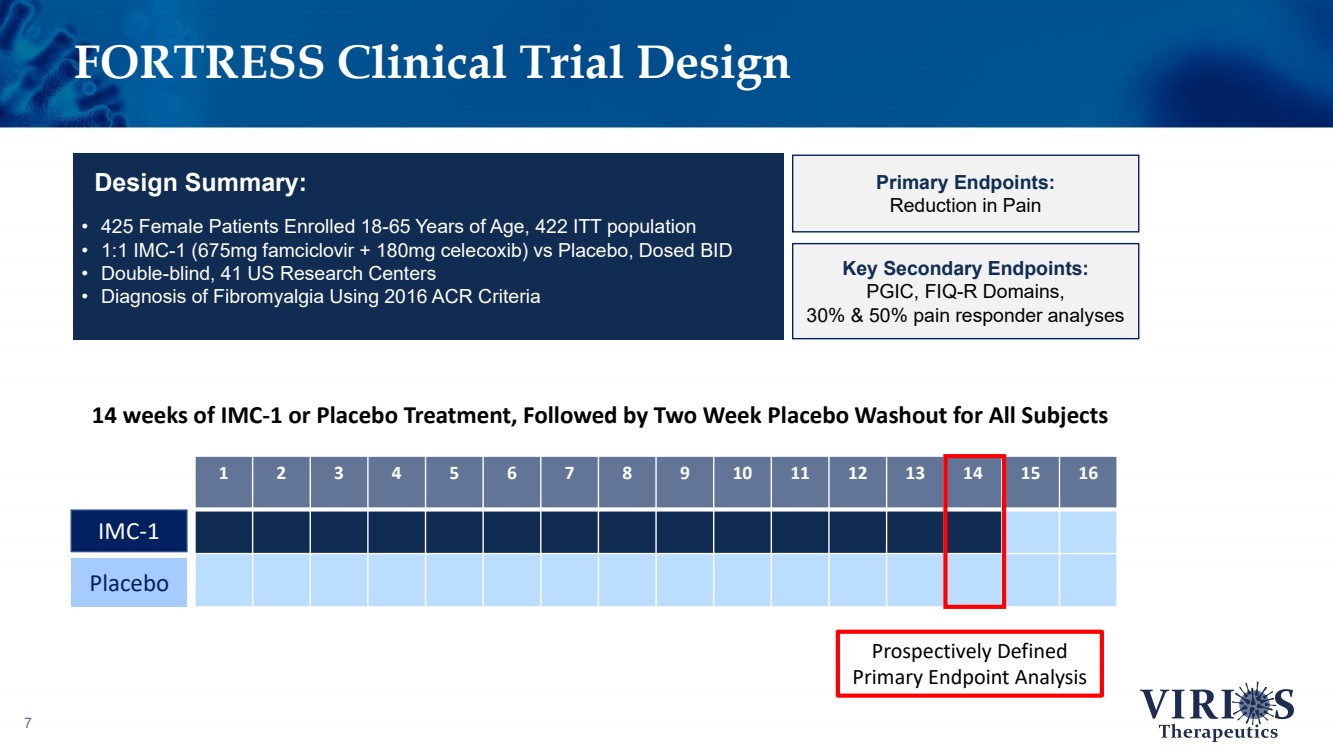
| FORTRESS Clinical Trial Design 7 • 425 Female Patients Enrolled 18 - 65 Years of Age, 422 ITT population • 1:1 IMC - 1 (675mg famciclovir + 180mg celecoxib) vs Placebo, Dosed BID • Double - blind, 41 US Research Centers • Diagnosis of Fibromyalgia Using 2016 ACR Criteria Design Summary: Primary Endpoints: Reduction in Pain Key Secondary Endpoints: PGIC, FIQ - R Domains, 30% & 50% pain responder analyses 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 14 weeks of IMC - 1 or Placebo Treatment, Followed by Two Week Placebo Washout for All Subjects IMC - 1 Placebo Prospectively Defined Primary Endpoint Analysis |
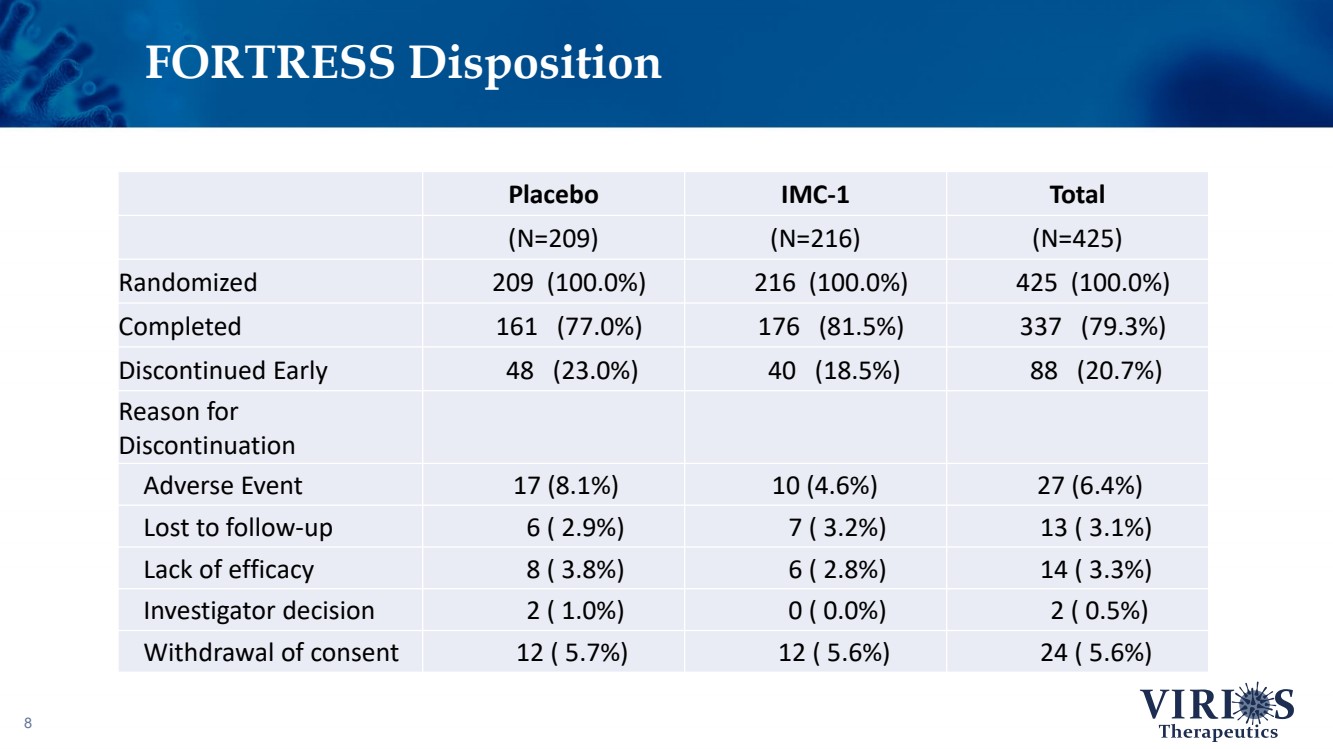
| FORTRESS Disposition 8 Placebo IMC - 1 Total (N=209) (N=216) (N=425) Randomized 209 (100.0%) 216 (100.0%) 425 (100.0%) Completed 161 (77.0%) 176 (81.5%) 337 (79.3%) Discontinued Early 48 (23.0%) 40 (18.5%) 88 (20.7%) Reason for Discontinuation Adverse Event 17 (8.1%) 10 (4.6%) 27 (6.4%) Lost to follow - up 6 ( 2.9%) 7 ( 3.2%) 13 ( 3.1%) Lack of efficacy 8 ( 3.8%) 6 ( 2.8%) 14 ( 3.3%) Investigator decision 2 ( 1.0%) 0 ( 0.0%) 2 ( 0.5%) Withdrawal of consent 12 ( 5.7%) 12 ( 5.6%) 24 ( 5.6%) |
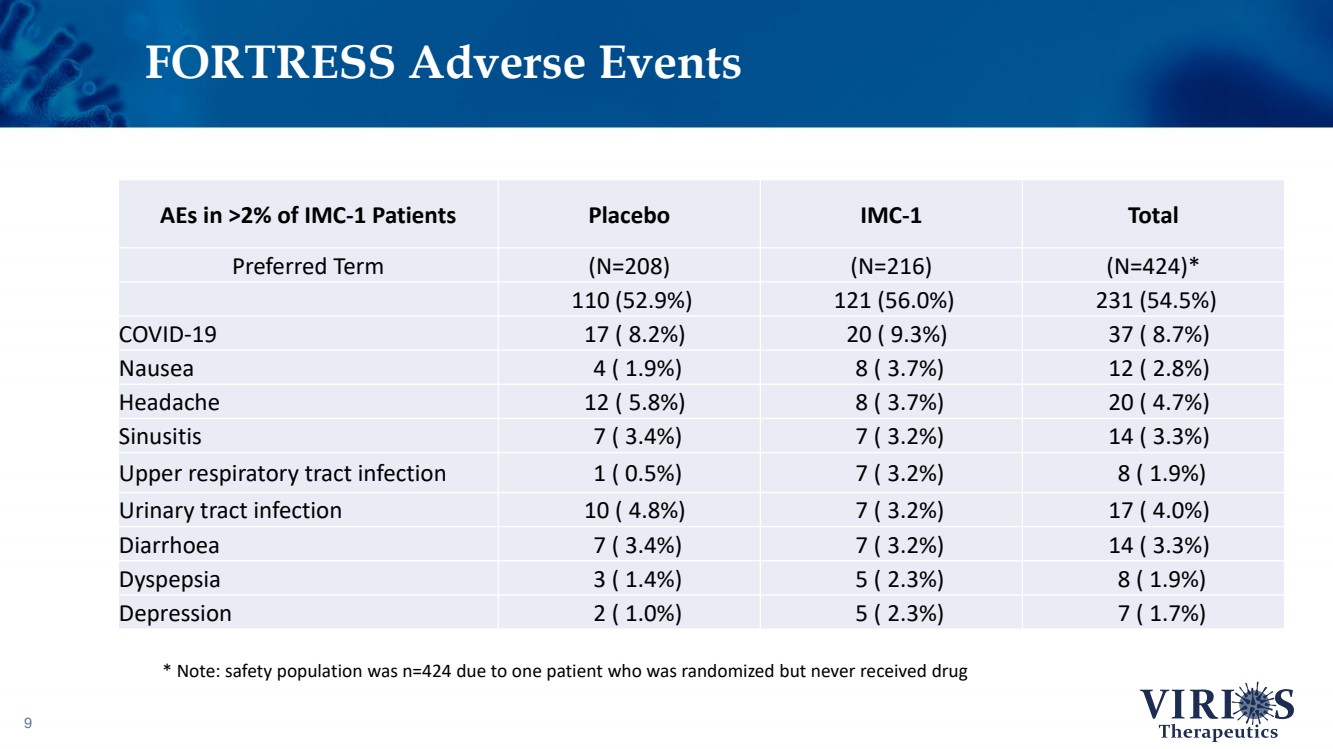
| FORTRESS Adverse Events 9 AEs in >2% of IMC - 1 Patients Placebo IMC - 1 Total Preferred Term (N=208) (N=216) (N=424)* 110 (52.9%) 121 (56.0%) 231 (54.5%) COVID - 19 17 ( 8.2%) 20 ( 9.3%) 37 ( 8.7%) Nausea 4 ( 1.9%) 8 ( 3.7%) 12 ( 2.8%) Headache 12 ( 5.8%) 8 ( 3.7%) 20 ( 4.7%) Sinusitis 7 ( 3.4%) 7 ( 3.2%) 14 ( 3.3%) Upper respiratory tract infection 1 ( 0.5%) 7 ( 3.2%) 8 ( 1.9%) Urinary tract infection 10 ( 4.8%) 7 ( 3.2%) 17 ( 4.0%) Diarrhoea 7 ( 3.4%) 7 ( 3.2%) 14 ( 3.3%) Dyspepsia 3 ( 1.4%) 5 ( 2.3%) 8 ( 1.9%) Depression 2 ( 1.0%) 5 ( 2.3%) 7 ( 1.7%) * Note: safety population was n=424 due to one patient who was randomized but never received drug |
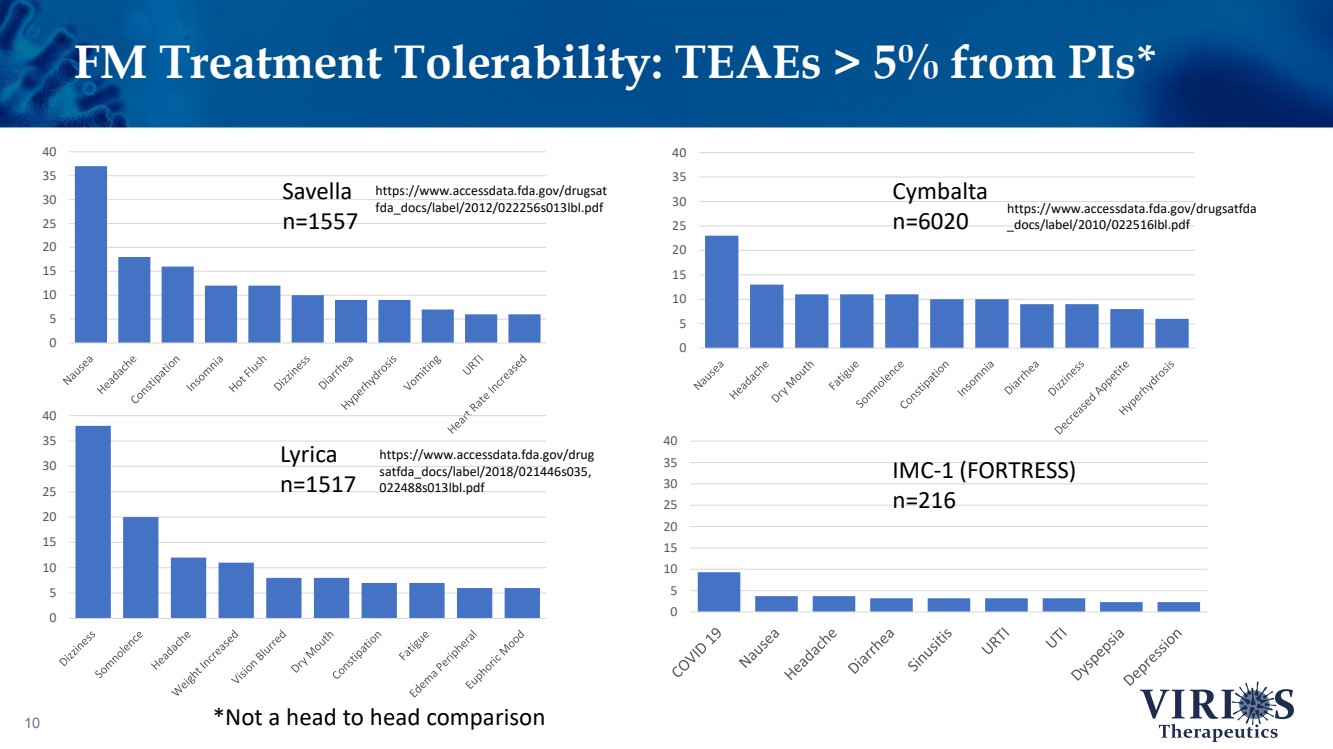
| FM Treatment Tolerability: TEAEs > 5% from PIs* 10 0 5 10 15 20 25 30 35 40 0 5 10 15 20 25 30 35 40 0 5 10 15 20 25 30 35 40 0 5 10 15 20 25 30 35 40 Savella n=1557 Cymbalta n=6020 Lyrica n=1517 IMC - 1 (FORTRESS) n=216 https://www.accessdata.fda.gov/drugsat fda_docs/label/2012/022256s013lbl.pdf https://www.accessdata.fda.gov/drugsatfda _docs/label/2010/022516lbl.pdf https://www.accessdata.fda.gov/drug satfda_docs/label/2018/021446s035, 022488s013lbl.pdf *Not a head to head comparison |
| Primary Endpoint: Diary Pain Improvement Over Time 11 |
| PROMIS Fatigue Improvement - Full Dataset 12 P=NS |
| 13 FIQR Symptoms & Function Improvement FIQR Symptom Domain FIQR Function Domain |
| Enrollment Timing and COVID - 19 Variants 14 https://www.nytimes.com/interactive/2021/health/coronavirus - variant - tracker.html Cohort 1 Jun 2021 – Nov 2 nd 2021 Cohort 2 Nov 3 rd 2021 - Apr 2022 |
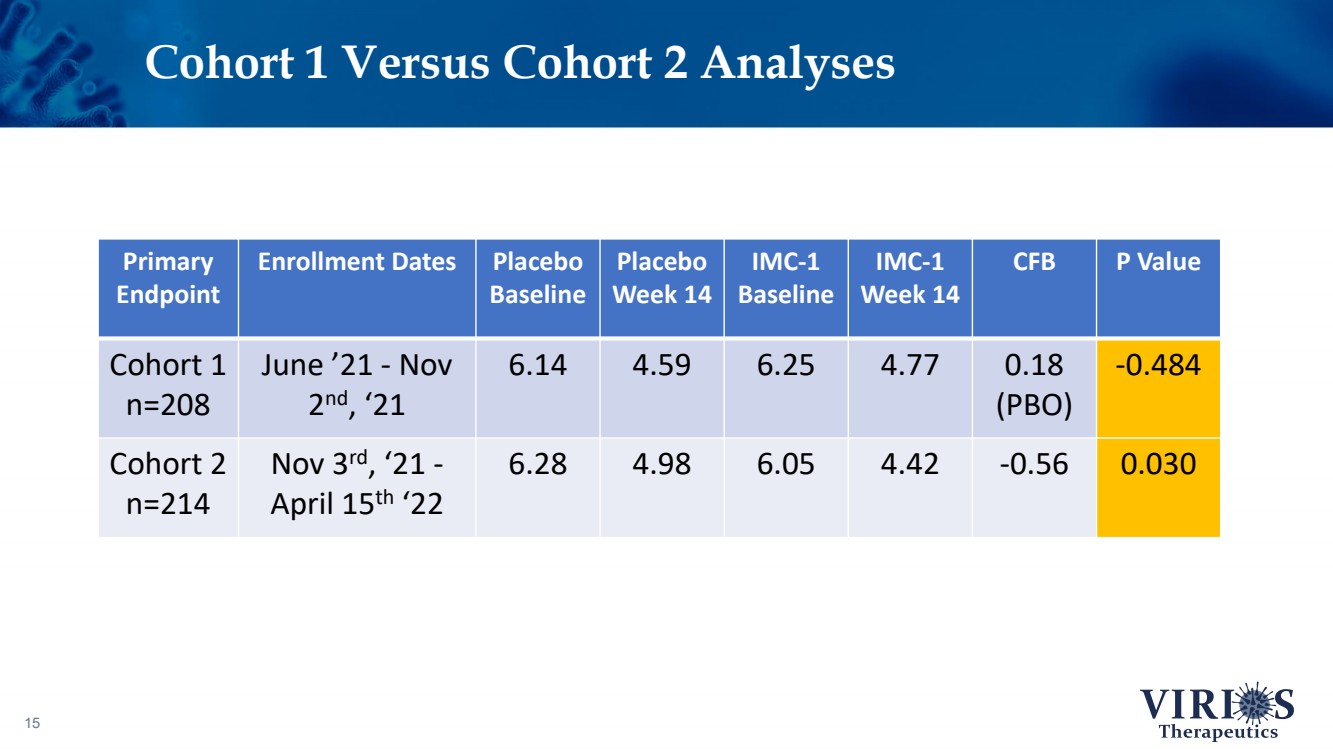
| Cohort 1 Versus Cohort 2 Analyses 15 Primary Endpoint Enrollment Dates Placebo Baseline Placebo Week 14 IMC - 1 Baseline IMC - 1 Week 14 CFB P Value Cohort 1 n=208 June ’21 - Nov 2 nd , ‘21 6.14 4.59 6.25 4.77 0.18 (PBO) - 0.484 Cohort 2 n=214 Nov 3 rd , ‘21 - April 15 th ‘22 6.28 4.98 6.05 4.42 - 0.56 0.030 |
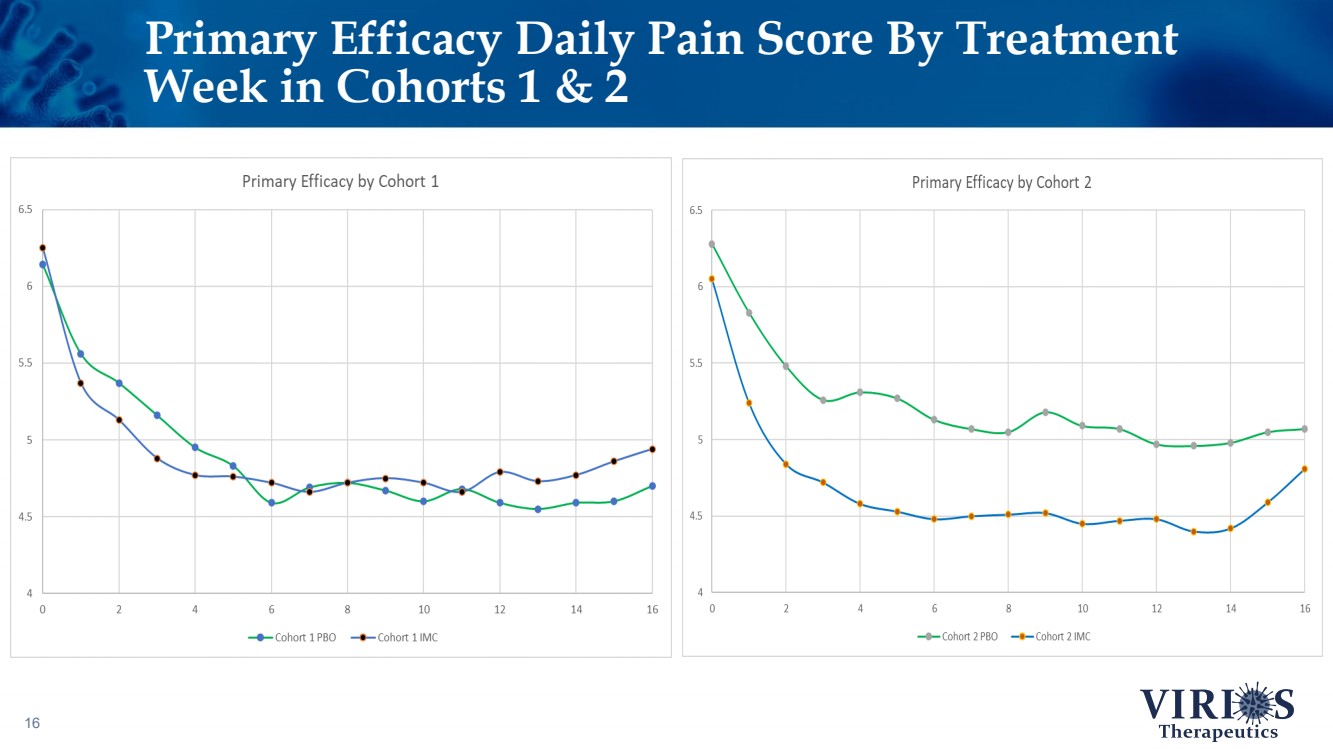
| Primary Efficacy Daily Pain Score By Treatment Week in Cohorts 1 & 2 16 |
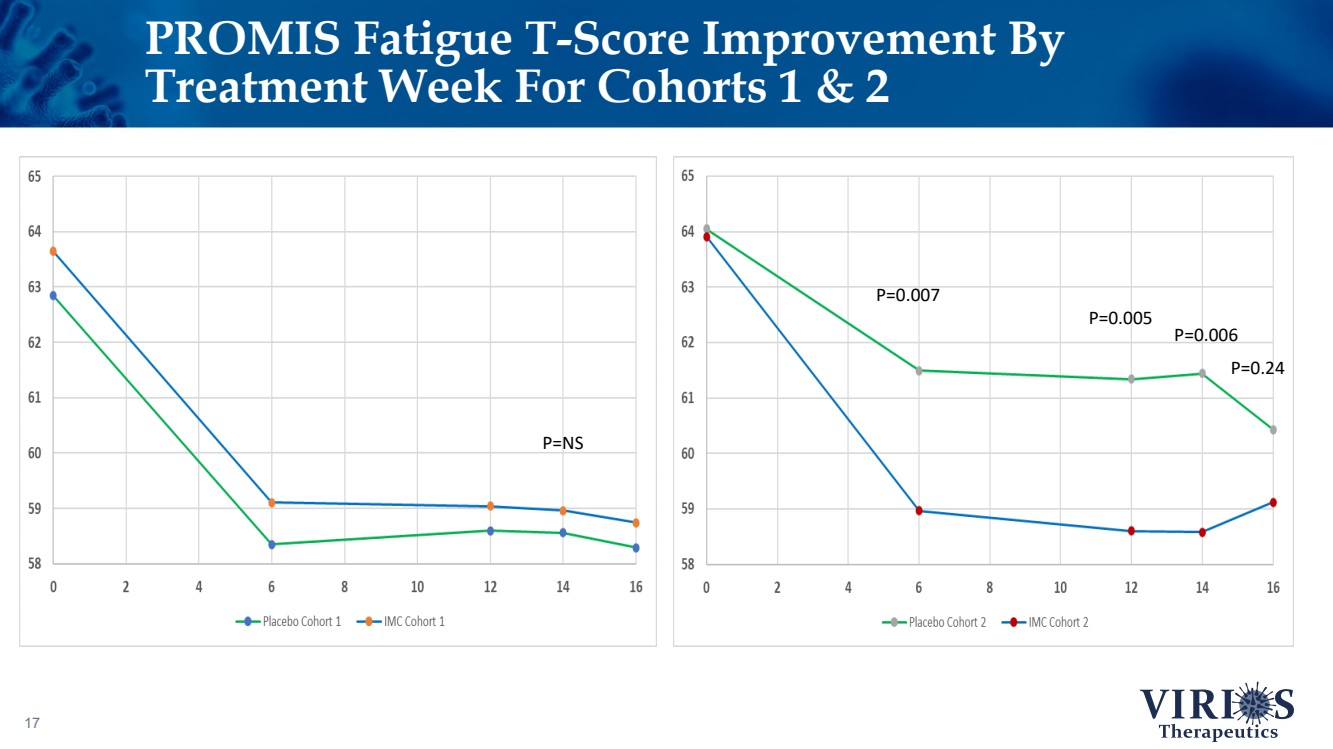
| PROMIS Fatigue T - Score Improvement By Treatment Week For Cohorts 1 & 2 17 P=NS P=0.007 P=0.005 P=0.006 P=0.24 |
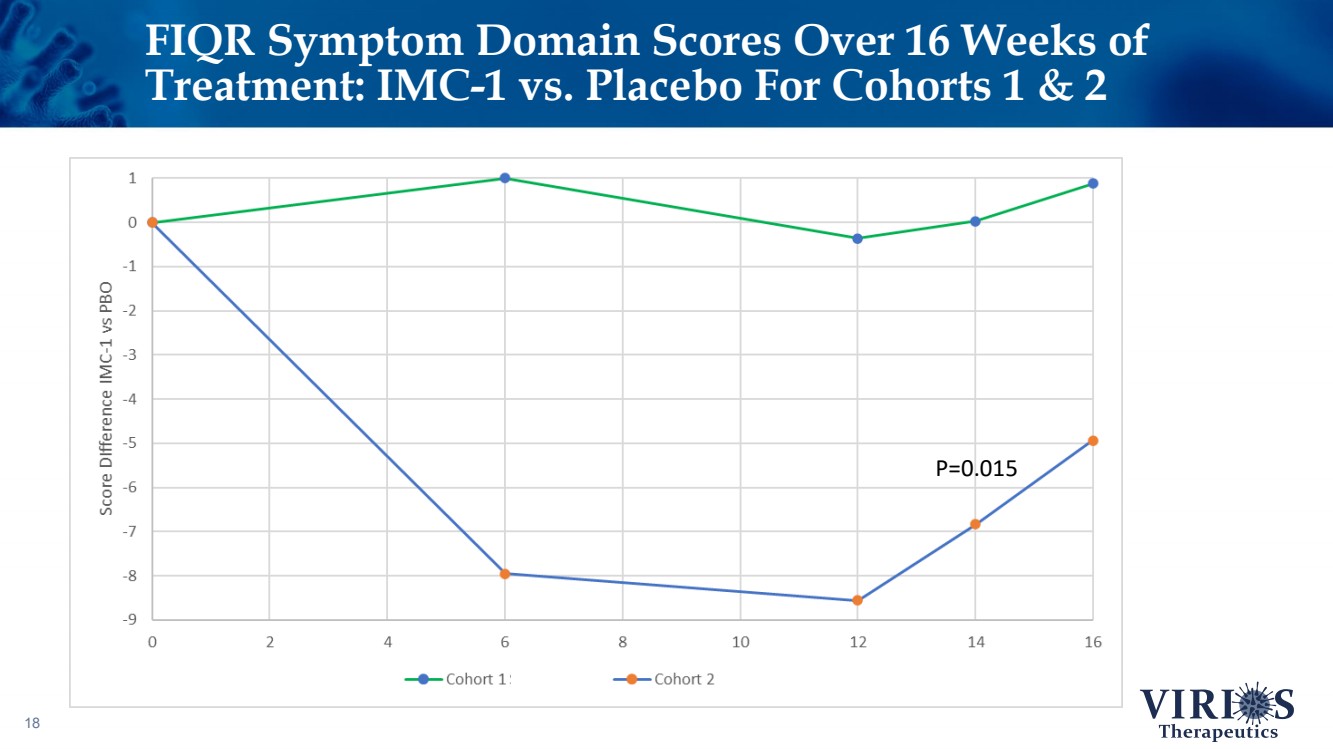
| FIQR Symptom Domain Scores Over 16 Weeks of Treatment: IMC - 1 vs. Placebo For Cohorts 1 & 2 18 P=0.015 |
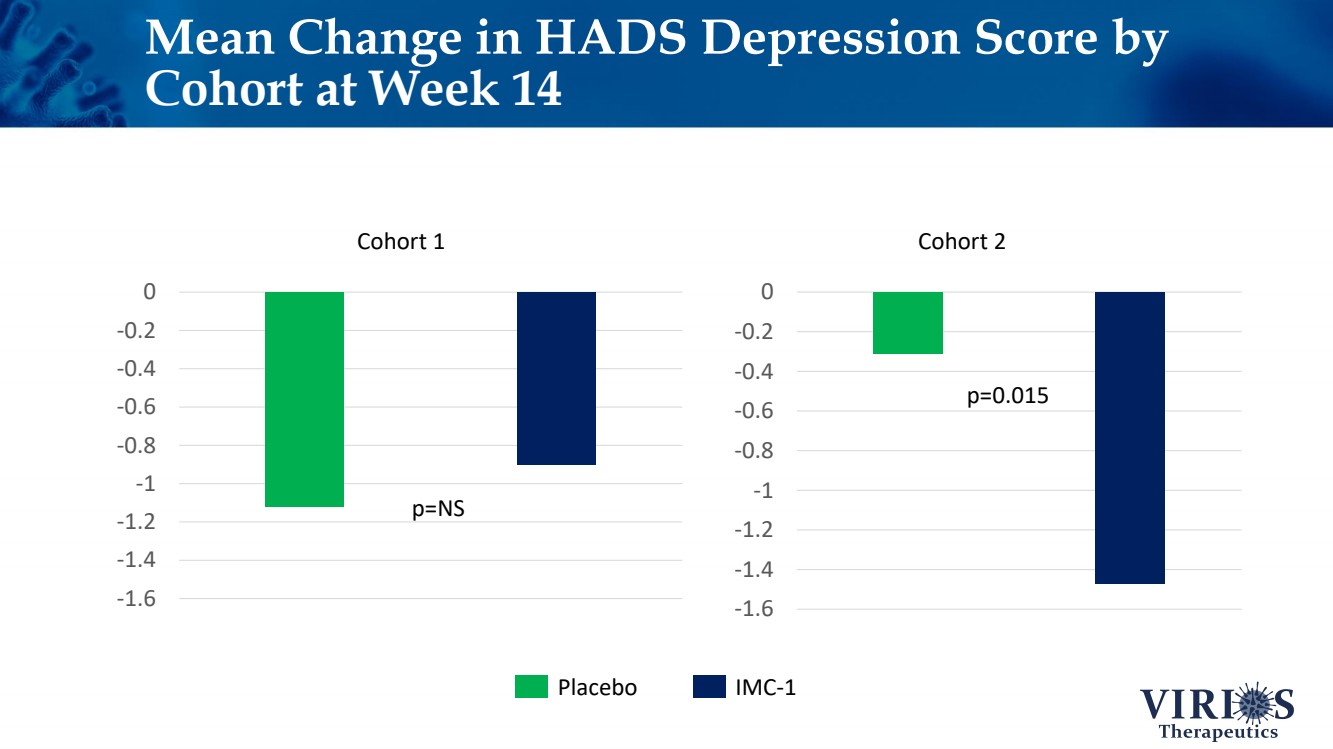
| -1.6 -1.4 -1.2 -1 -0.8 -0.6 -0.4 -0.2 0 -1.6 -1.4 -1.2 -1 -0.8 -0.6 -0.4 -0.2 0 Mean Change in HADS Depression Score by Cohort at Week 14 Cohort 1 Cohort 2 IMC - 1 Placebo p=0.015 p=NS |
| FORTRESS Summary 20 ❖ Virios management strongly believes this mechanism has potential to improve FM patient care – Positive Phase 2a clinical study results – IMC - 1 in Cohort 2 delivered statistically significant improvement in FM patient pain, fatigue, depression and overall health status – IMC - 1 in Cohort 2 efficacy results were consistent with the expected profile from previous Phase 2a study – The difference in results between Cohort 1 and Cohort 2 is highly unlikely due to chance ❖ We believe the excellent overall safety and tolerability profile observed in FORTRESS supports future product development ❖ Our ultimate goal is to get IMC - 1 to market ❖ Our short - term plan is to engage with KOLs/ BoD to better understand the Phase 2b data and design a forward development plan to maximize the potential of IMC - 1 |
| HSV - 1 virus THANK YOU! www.virios.com For Additional Information, Contact: Kirin Smith : ksmith@pcgadvisory.com or ir@virios.com |