| NASDAQ: DWTX Q4 2025 Update Deep Commitment to Advancing First-in-Class New Therapeutic Treatments for Cancer Patients Suffering from Neuropathy |
| NASDAQ: DWTX CONFIDENTIAL – Dogwood Therapeutics, Inc. 2 Forward-Looking Statements and Disclaimers Forward-Looking Statements Statements in this presentation contain “forward-looking statements,” within the meaning of the U.S. Private Securities Litigation Reform Act of 1995, that are subject to substantial risks and uncertainties. All statements, other than statements of historical fact, contained in this presentation are forward-looking statements. Forward-looking statements contained in this presentation may be identified by the use of words such as “anticipate,” “believe,” “contemplate,” “could,” “estimate,” “expect,” “intend,” “seek,” “may,” “might,” “plan,” “potential,” “predict,” “project,” “suggest,” “target,” “aim,” “should,” "will,” “would,” or the negative of these words or other similar expressions, although not all forward-looking statements contain these words. Forward-looking statements are based on the current expectations of Dogwood Therapeutics, Inc. (“Dogwood”) and are subject to inherent uncertainties, risks and assumptions that are difficult to predict, including risks related to the completion, timing and results of current and future clinical studies relating to Dogwood’s product candidates. Further, certain forward-looking statements are based on assumptions as to future events that may not prove to be accurate. These and other risks and uncertainties are described more fully in the section titled “Risk Factors” in the Annual Report on Form 10-K for the year ended December 31, 2024, filed with the Securities and Exchange Commission. Forward-looking statements contained in this presentation are made as of this date, and Dogwood undertakes no duty to update such information except as required under applicable law. Important Additional Information and Where to Find Additional Information Dogwood, its directors and certain of its executive officers are deemed to be participants in the solicitation of proxies from Dogwood stockholders in connection with Dogwood's expected special meeting seeking stockholder approval of conversion of Dogwood’s preferred stock (“Preferred Stock”) and other matters related to the business combination with Wex Pharmaceuticals, Inc. (the “Combination”.) Information regarding the names of Dogwood’s directors and executive officers and their respective interests in Dogwood by security holdings or otherwise can be found in Dogwood’s proxy statement for its 2025 Annual Meeting of Stockholders, filed with the SEC on April 30, 2025. To the extent holdings of Dogwood’s securities have changed since the amounts set forth in Dogwood’s proxy statement for the 2025 Annual Meeting of Stockholders, such changes have been or will be reflected on Initial Statements of Beneficial Ownership on Form 3 or Statements of Change in Ownership on Form 4 filed with the SEC. These documents are available free of charge at the SEC’s website at www.sec.gov. Dogwood intends to file a definitive proxy statement and accompanying proxy card with the SEC in connection with the solicitation of proxies from Dogwood stockholders in connection with Dogwood's expected special meeting seeking stockholder approval of conversion of the Preferred Stock and other matters related to the Combination. Additional information regarding the identity of participants, and their direct or indirect interests, by security holdings or otherwise, will be set forth in Dogwood’s proxy statement for such special meeting, including the schedules and appendices thereto. INVESTORS AND STOCKHOLDERS ARE STRONGLY ENCOURAGED TO READ ANY SUCH PROXY STATEMENT AND THE ACCOMPANYING PROXY CARD AND ANY AMENDMENTS AND SUPPLEMENTS THERETO AS WELL AS ANY OTHER DOCUMENTS FILED BY DOGWOOD WITH THE SEC CAREFULLY AND IN THEIR ENTIRETY WHEN THEY BECOME AVAILABLE AS THEY WILL CONTAIN IMPORTANT INFORMATION. Stockholders will be able to obtain copies of the proxy statement, any amendments or supplements to the proxy statement, the accompanying proxy card, and other documents filed by Dogwood with the SEC for no charge at the SEC’s website at www.sec.gov. Copies will also be available at no charge at the Investor Relations section of Dogwood’s corporate website at https://ir.DWTX.com/ or by contacting Dogwood’s Investor Relations at Dogwood Therapeutics, Inc., 44 Milton Avenue, Alpharetta, GA 30009 or by emailing Dogwood’s Investor Relations at IR@dwtx.com or by calling (866) 620-8655. This presentation may contain market, industry and other data obtained from third party resources. Dogwood believes the data from such third-party sources is reliable, however it has not independently verified any of such data and cannot guarantee its accuracy or completeness. Similarly, internal research and forecasts, which Dogwood believes to be reliable based upon management’s knowledge of the market and the industry, have not been verified by any independent sources. While Dogwood is not aware of any misstatements regarding the market or industry data presented herein, the estimates involve risks and uncertainties and are subject to change based on various factors and evolution over time. Dogwood owns or has rights to use a number of registered and common law trademarks, service marks and/or trade names in connection with our business in the United States and/or in certain foreign jurisdictions. Solely for convenience, the trademarks, service marks, logos and trade names referred to in this presentation may be referred to without the ® and symbols, but such references are not intended to indicate, in any way, that Dogwood will not assert, to the fullest extent under applicable law, its rights or the rights of the applicable licensors to these trademarks, service marks and trade names. |
| NASDAQ: DWTX CONFIDENTIAL – Dogwood Therapeutics, Inc. 3 Dogwood is Led by an Executive Team with Extensive Drug Development and Commercialization Experience DWTX Executive Team Greg Duncan Chairman & CEO R. Michael Gendreau MD, PhD CMO Ralph Grosswald SVP of Operations Angela Walsh CFO Management’s Brand Development & Commercialization Experience Includes: |
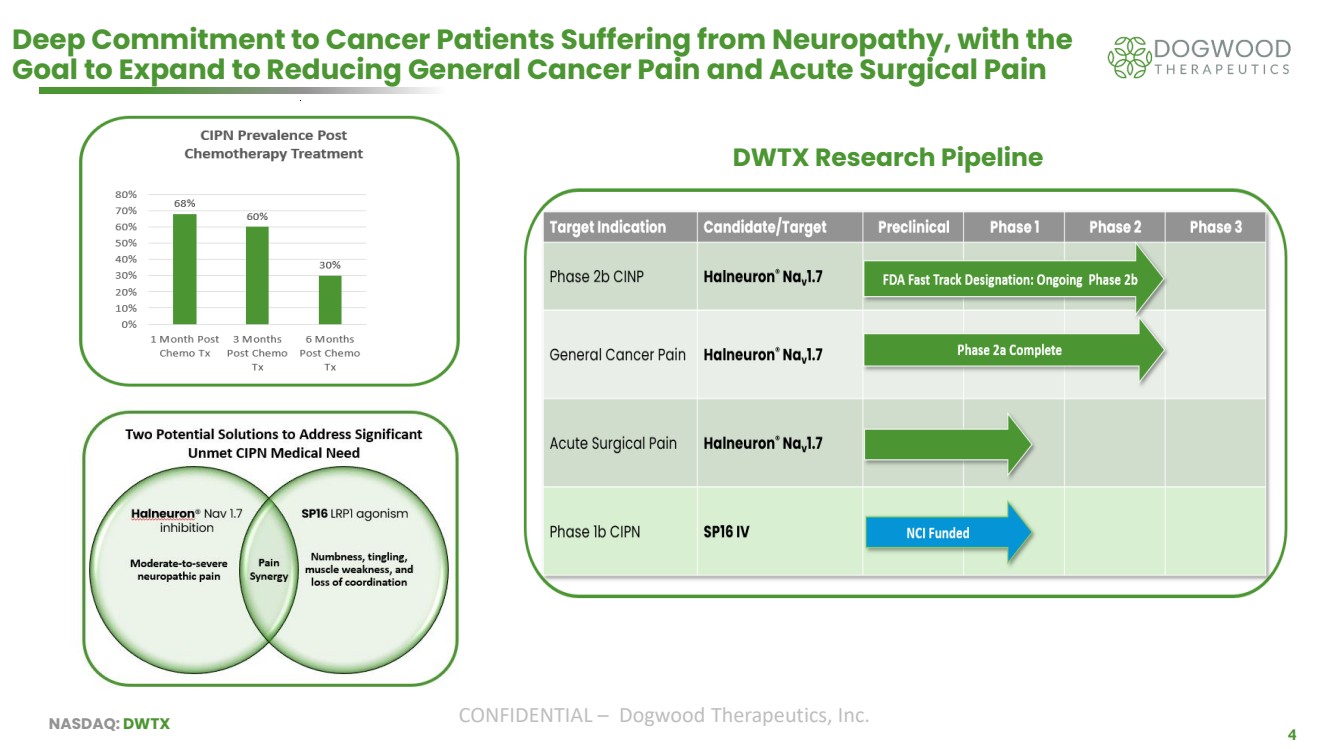
| NASDAQ: DWTX CONFIDENTIAL – Dogwood Therapeutics, Inc. 4 Deep Commitment to Cancer Patients Suffering from Neuropathy, with the Goal to Expand to Reducing General Cancer Pain and Acute Surgical Pain DWTX Research Pipeline |
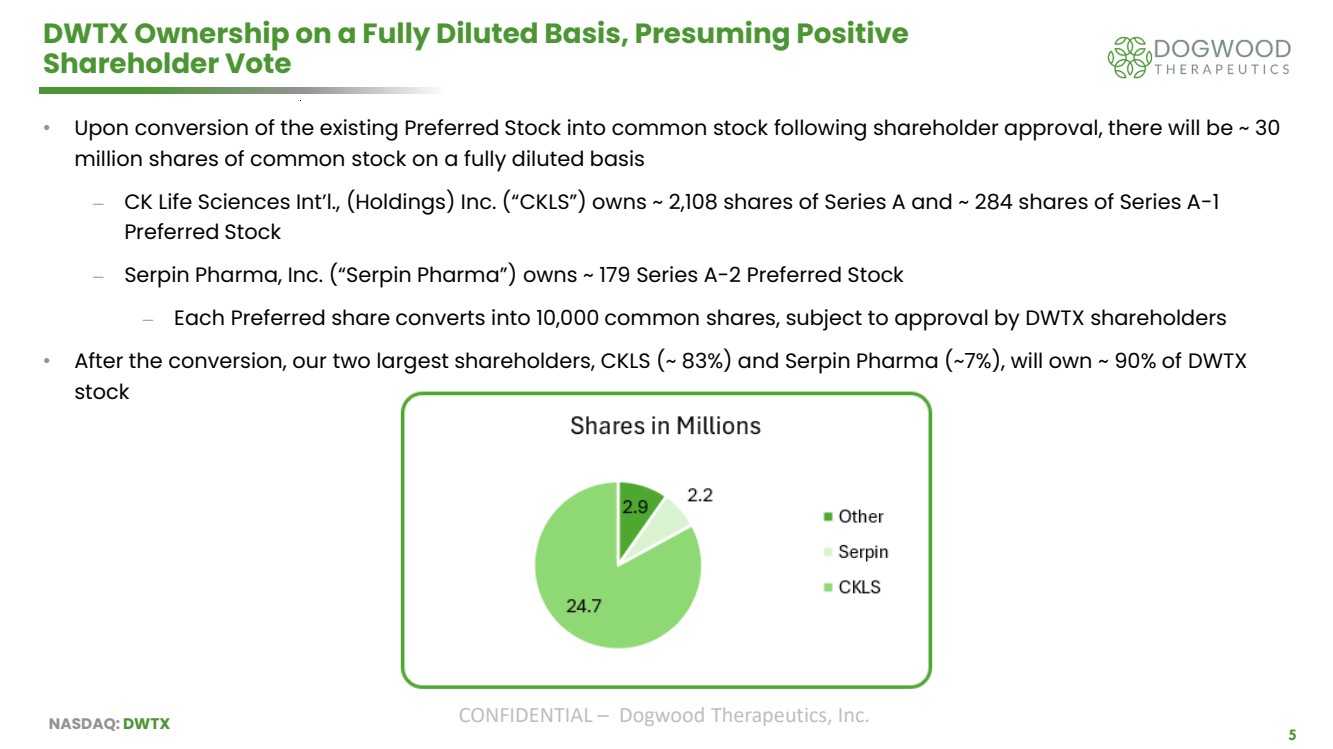
| NASDAQ: DWTX CONFIDENTIAL – Dogwood Therapeutics, Inc. 5 DWTX Ownership on a Fully Diluted Basis, Presuming Positive Shareholder Vote • Upon conversion of the existing Preferred Stock into common stock following shareholder approval, there will be ~ 30 million shares of common stock on a fully diluted basis ⎼ CK Life Sciences Int’l., (Holdings) Inc. (“CKLS”) owns ~ 2,108 shares of Series A and ~ 284 shares of Series A-1 Preferred Stock ⎼ Serpin Pharma, Inc. (“Serpin Pharma”) owns ~ 179 Series A-2 Preferred Stock ⎼ Each Preferred share converts into 10,000 common shares, subject to approval by DWTX shareholders • After the conversion, our two largest shareholders, CKLS (~ 83%) and Serpin Pharma (~7%), will own ~ 90% of DWTX stock |
| CONFIDENTIAL – Dogwood Therapeutics, Inc. Halneuron® Research Program Overview |
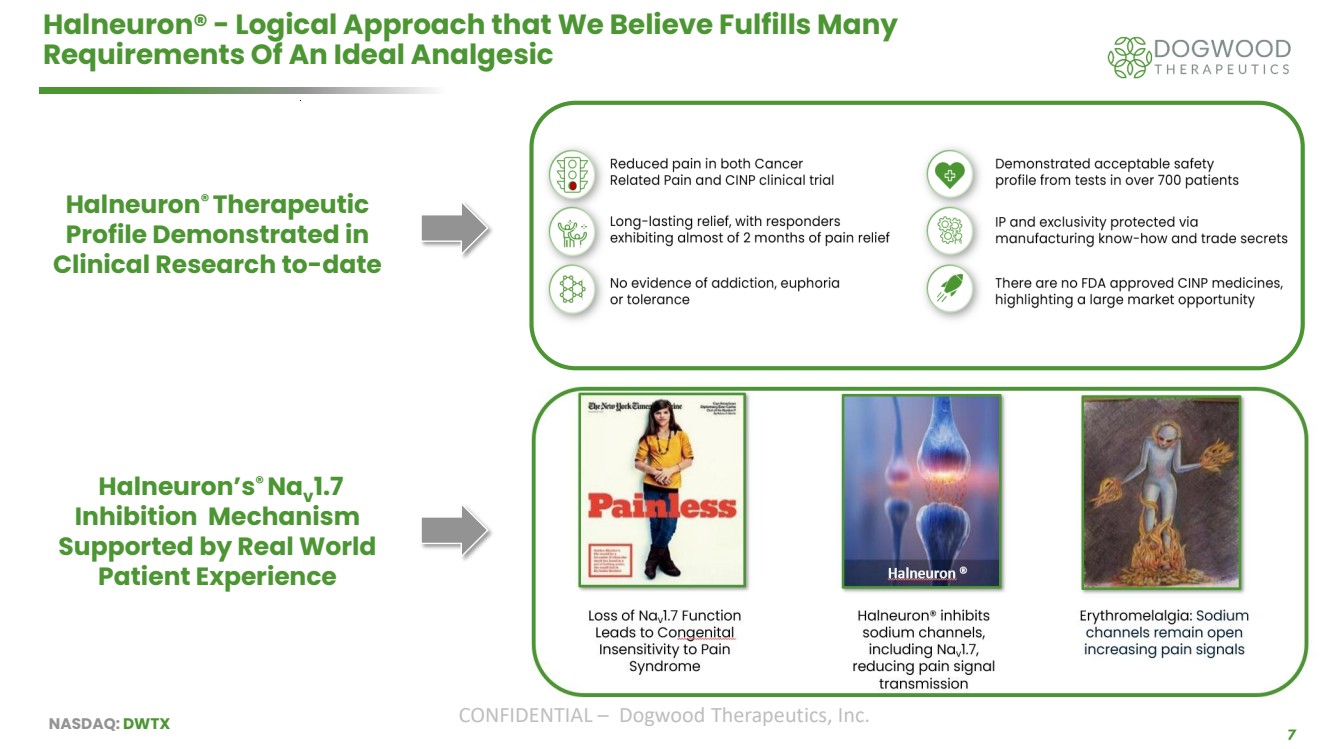
| NASDAQ: DWTX CONFIDENTIAL – Dogwood Therapeutics, Inc. 7 Halneuron® - Logical Approach that We Believe Fulfills Many Requirements Of An Ideal Analgesic Halneuron® Therapeutic Profile Demonstrated in Clinical Research to-date Halneuron’s® Nav 1.7 Inhibition Mechanism Supported by Real World Patient Experience |
| NASDAQ: DWTX CONFIDENTIAL – Dogwood Therapeutics, Inc. 8 CINP Represents a Major Unmet Medical Need • Approximately 20M new patients were diagnosed with cancer worldwide in 2022 ⎼ ~2M new cancer cases in the US in 2025 ⎼ ~40% of cancer patients live with chronic pain • Over 50% of cancer patients are treated annually with chemotherapy • CINP is nerve damage caused by certain chemotherapy drugs, leading to a range of neuropathic symptoms, including pain, numbness, and tingling, often in the hands and feet ⎼ CINP severity characterized as mild (25%), moderate (50%), or severe (25%) across most markets • Estimates suggest almost 70% of patients treated with chemotherapy experience CINP ⎼ 30% of chemotherapy treated patients continue to experience CINP six months post treatment • Chemotherapy utilization is expected to increase by 54% by 2040 • Up to six in ten CINP patients are likely to be treated with opioids in 2025 ⎼ Cancer patients using opioids develop clinically significant adverse effects (e.g. cognitive impairment and hallucinations, as well as constipation or nausea and vomiting) Sources: American Cancer Society; 2024, WHO, 2024; Lancet Oncology, 2019; DelveInsight, 2018; de Stoutz ND, Bruera E, Suarez-Almazor M, J Pain Symptom Manage 1995; Trescot AM, Boswell MV, Atluri SL, et al., Pain Physician 2006 |
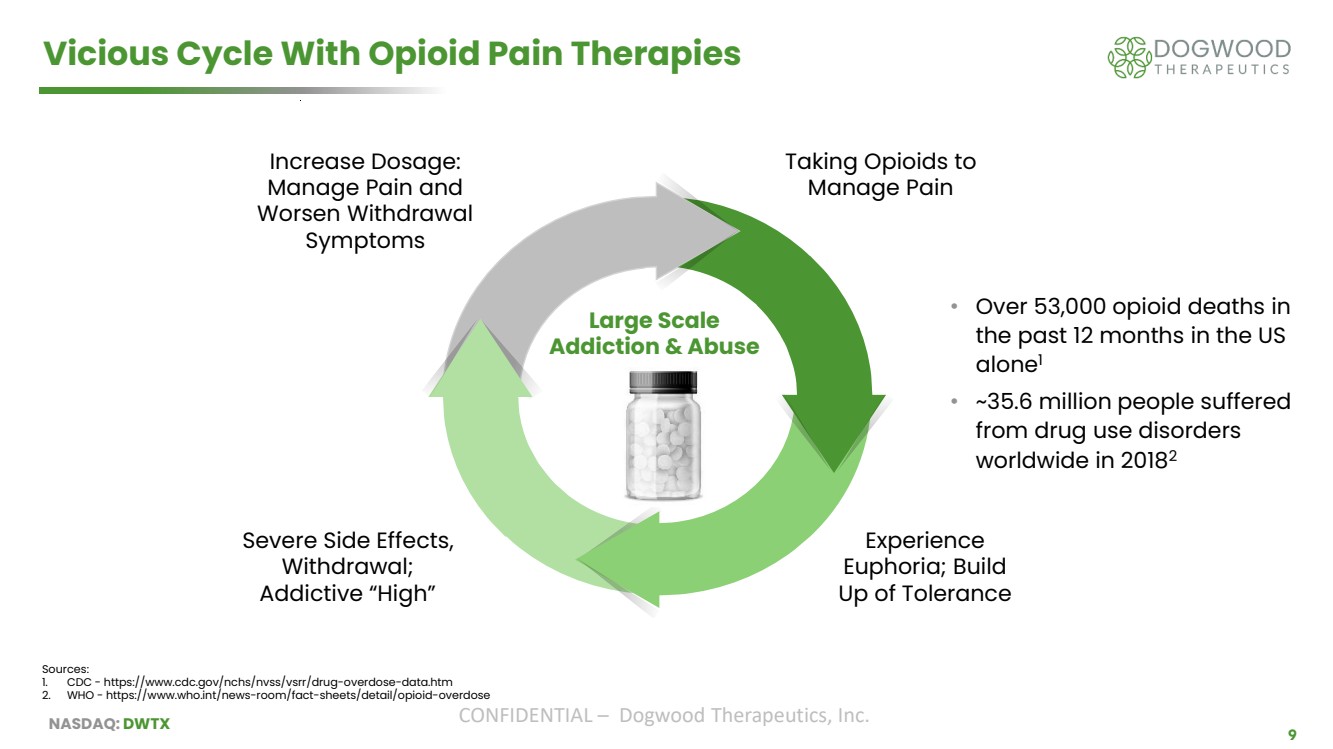
| NASDAQ: DWTX CONFIDENTIAL – Dogwood Therapeutics, Inc. 9 Vicious Cycle With Opioid Pain Therapies Taking Opioids to Manage Pain Experience Euphoria; Build Up of Tolerance Severe Side Effects, Withdrawal; Addictive “High” Increase Dosage: Manage Pain and Worsen Withdrawal Symptoms Large Scale Addiction & Abuse • Over 53,000 opioid deaths in the past 12 months in the US alone1 • ~35.6 million people suffered from drug use disorders worldwide in 20182 Sources: 1. CDC - https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm 2. WHO - https://www.who.int/news-room/fact-sheets/detail/opioid-overdose |
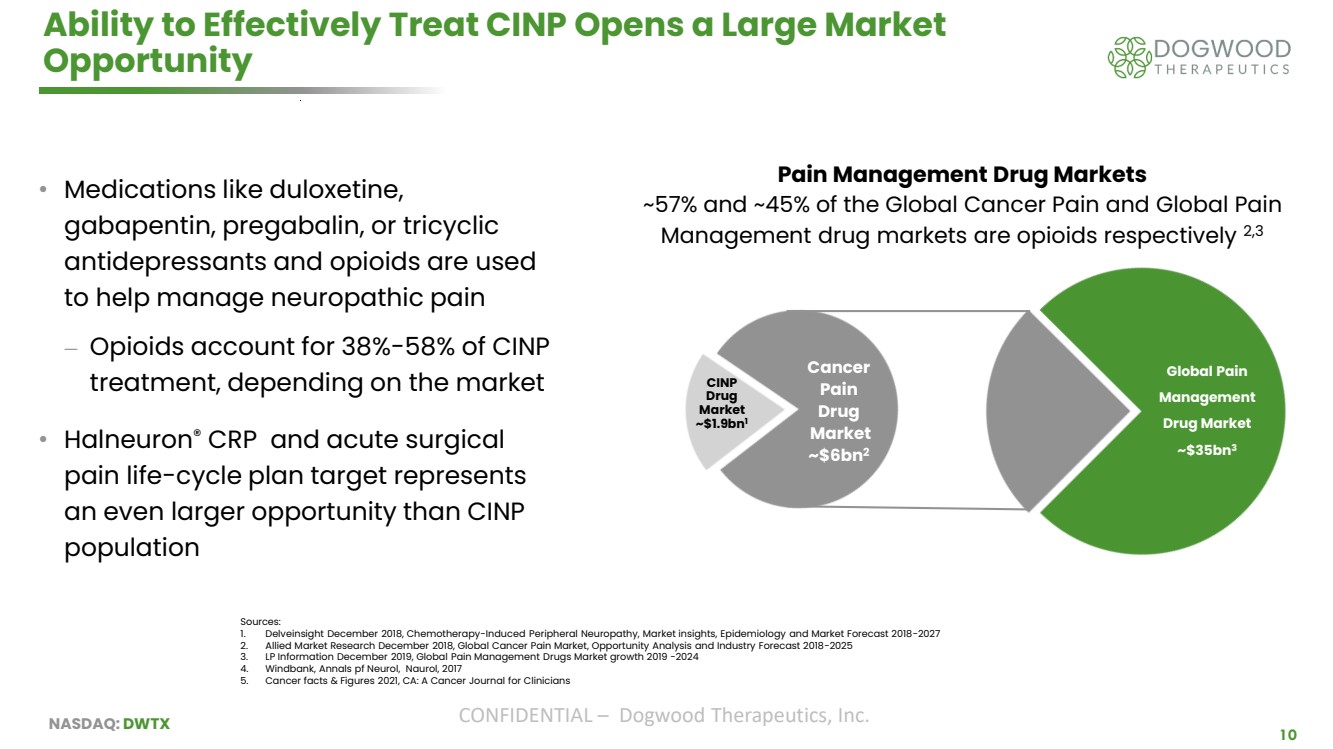
| NASDAQ: DWTX CONFIDENTIAL – Dogwood Therapeutics, Inc. 10 Ability to Effectively Treat CINP Opens a Large Market Opportunity • Medications like duloxetine, gabapentin, pregabalin, or tricyclic antidepressants and opioids are used to help manage neuropathic pain ⎼ Opioids account for 38%-58% of CINP treatment, depending on the market • Halneuron® CRP and acute surgical pain life-cycle plan target represents an even larger opportunity than CINP population Sources: 1. Delveinsight December 2018, Chemotherapy-Induced Peripheral Neuropathy, Market insights, Epidemiology and Market Forecast 2018-2027 2. Allied Market Research December 2018, Global Cancer Pain Market, Opportunity Analysis and Industry Forecast 2018-2025 3. LP Information December 2019, Global Pain Management Drugs Market growth 2019 -2024 4. Windbank, Annals pf Neurol, Naurol, 2017 5. Cancer facts & Figures 2021, CA: A Cancer Journal for Clinicians Global Pain Management Drug Market ~$35bn3 Cancer Pain Drug Market ~$6bn2 CINP Drug Market ~$1.9bn1 Pain Management Drug Markets ~57% and ~45% of the Global Cancer Pain and Global Pain Management drug markets are opioids respectively 2,3 |
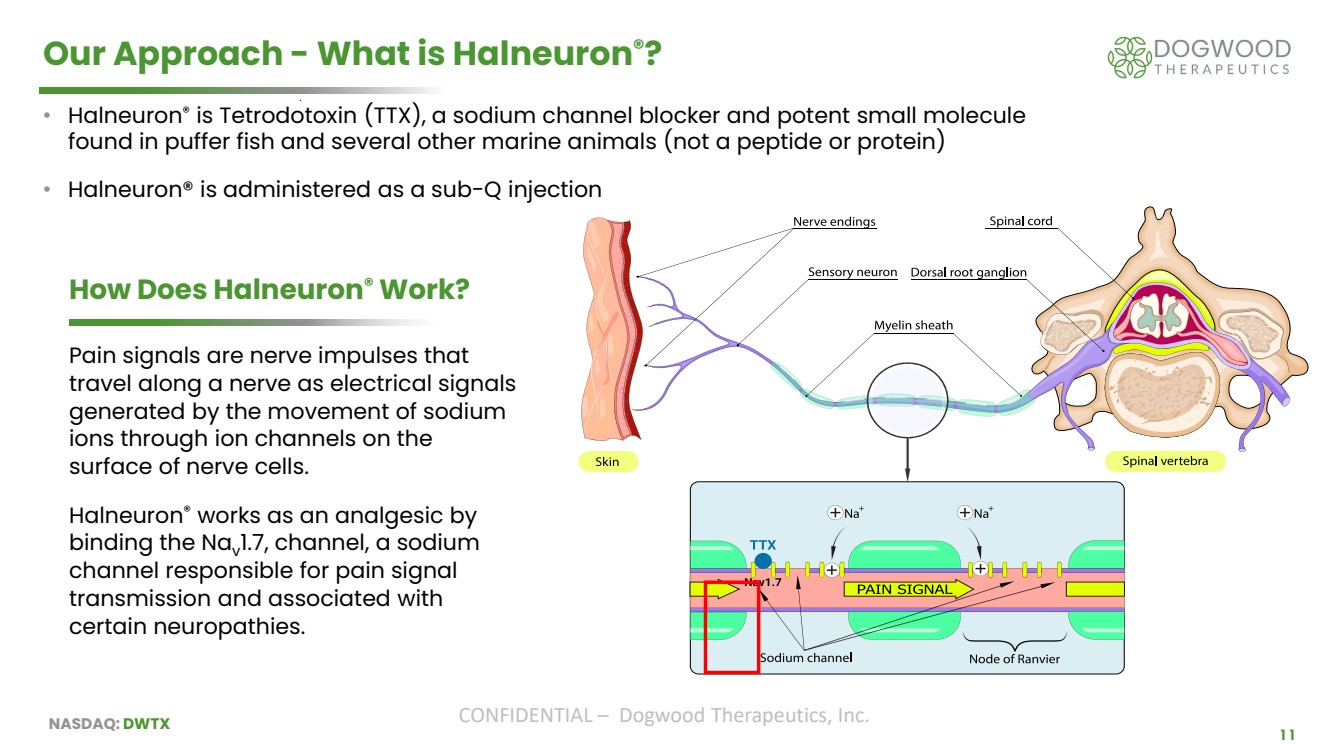
| NASDAQ: DWTX CONFIDENTIAL – Dogwood Therapeutics, Inc. 11 Our Approach - What is Halneuron®? • Halneuron® is Tetrodotoxin (TTX), a sodium channel blocker and potent small molecule found in puffer fish and several other marine animals (not a peptide or protein) • Halneuron® is administered as a sub-Q injection How Does Halneuron® Work? Pain signals are nerve impulses that travel along a nerve as electrical signals generated by the movement of sodium ions through ion channels on the surface of nerve cells. Halneuron® works as an analgesic by binding the Nav 1.7, channel, a sodium channel responsible for pain signal transmission and associated with certain neuropathies. |
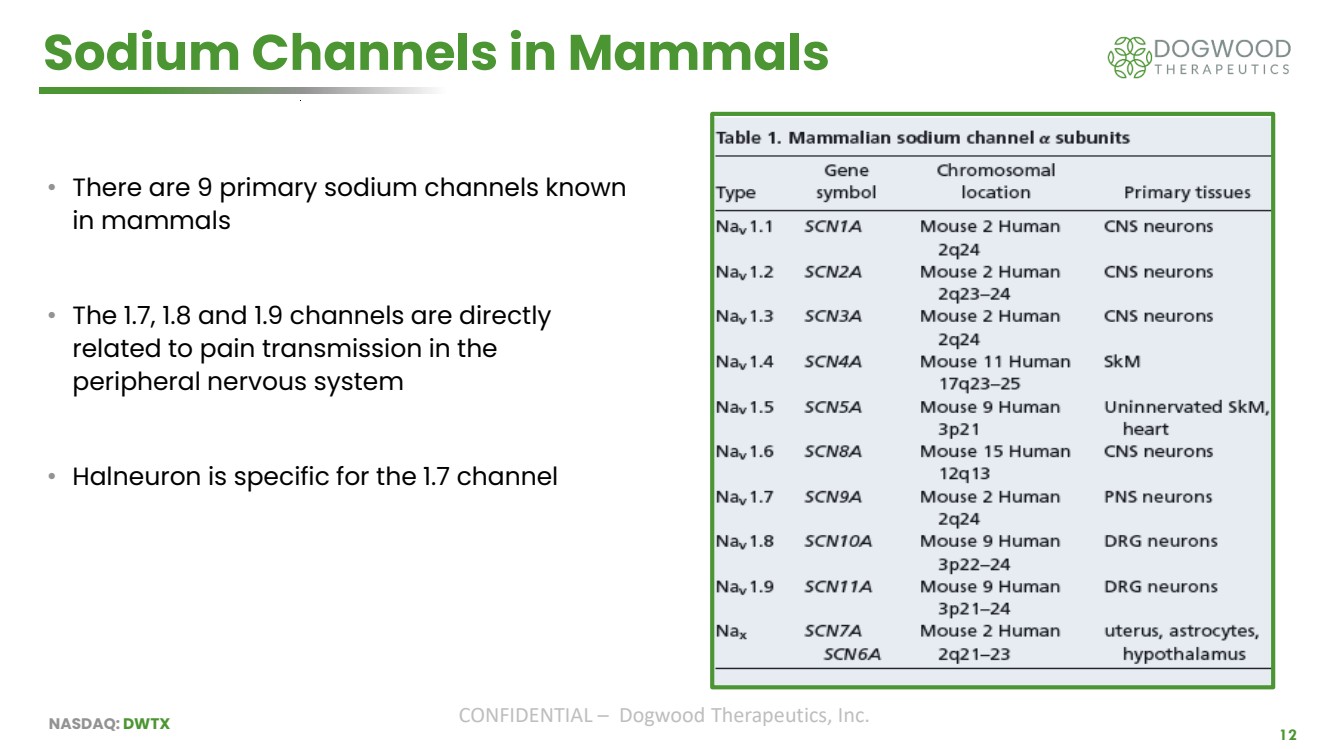
| NASDAQ: DWTX CONFIDENTIAL – Dogwood Therapeutics, Inc. Sodium Channels in Mammals • There are 9 primary sodium channels known in mammals • The 1.7, 1.8 and 1.9 channels are directly related to pain transmission in the peripheral nervous system • Halneuron is specific for the 1.7 channel 12 |
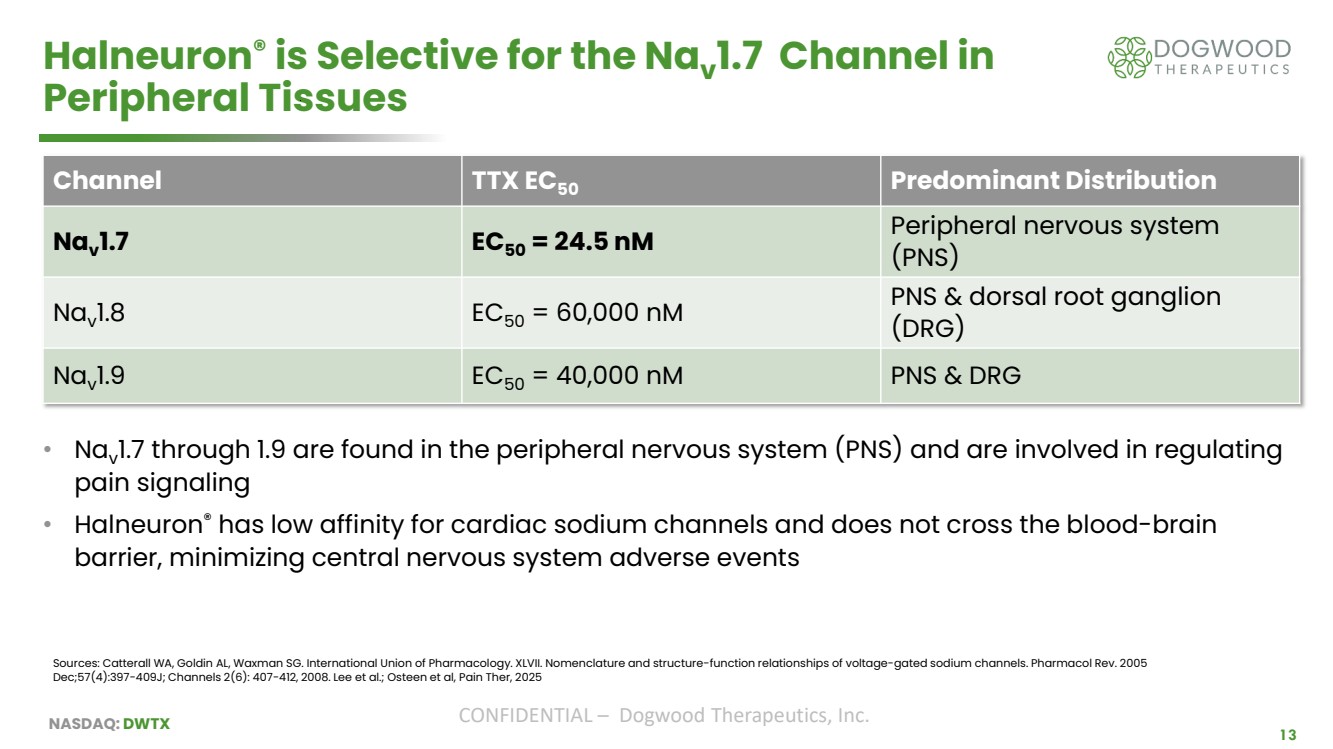
| NASDAQ: DWTX CONFIDENTIAL – Dogwood Therapeutics, Inc. 13 Halneuron® is Selective for the Nav 1.7 Channel in Peripheral Tissues • Nav 1.7 through 1.9 are found in the peripheral nervous system (PNS) and are involved in regulating pain signaling • Halneuron® has low affinity for cardiac sodium channels and does not cross the blood-brain barrier, minimizing central nervous system adverse events Sources: Catterall WA, Goldin AL, Waxman SG. International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol Rev. 2005 Dec;57(4):397-409J; Channels 2(6): 407-412, 2008. Lee et al.; Osteen et al, Pain Ther, 2025 Channel TTX EC50 Predominant Distribution Nav 1.7 EC50 = 24.5 nM Peripheral nervous system (PNS) Nav 1.8 EC50 = 60,000 nM PNS & dorsal root ganglion (DRG) Nav 1.9 EC50 = 40,000 nM PNS & DRG |
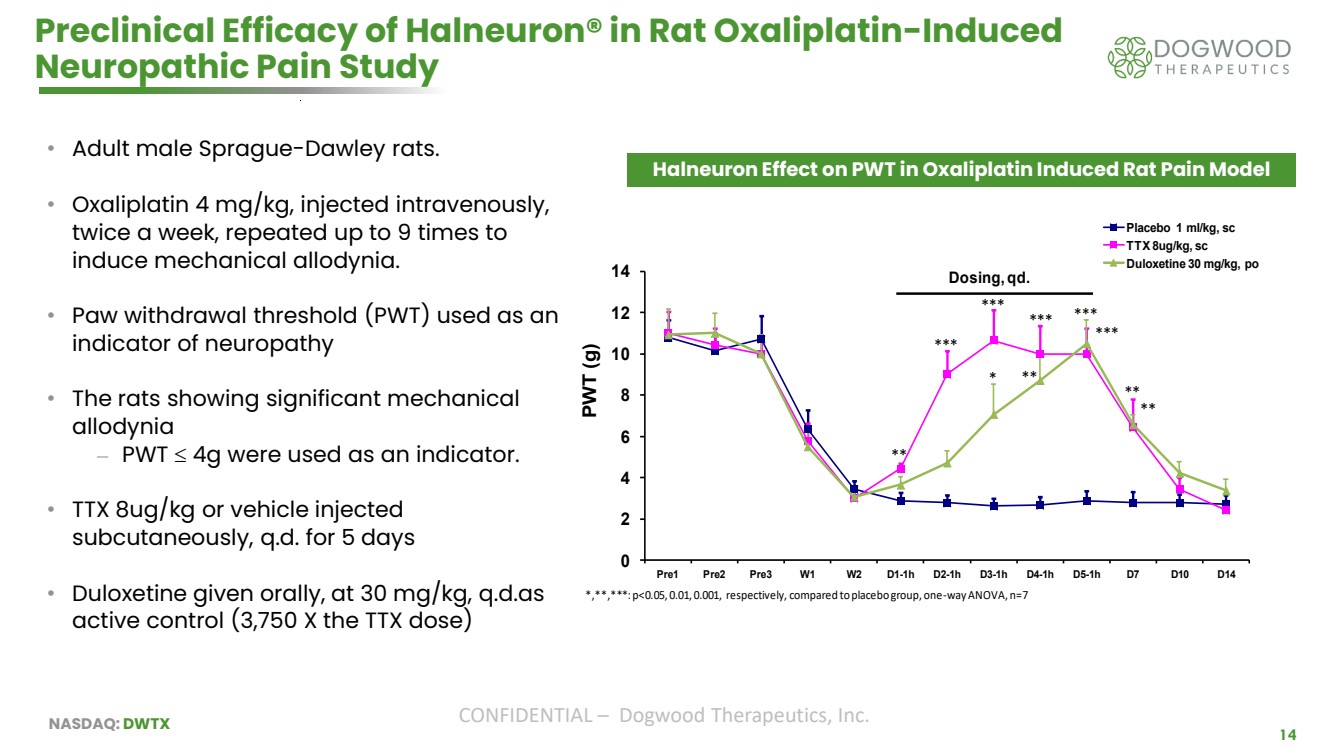
| NASDAQ: DWTX CONFIDENTIAL – Dogwood Therapeutics, Inc. 14 Preclinical Efficacy of Halneuron® in Rat Oxaliplatin-Induced Neuropathic Pain Study • Adult male Sprague-Dawley rats. • Oxaliplatin 4 mg/kg, injected intravenously, twice a week, repeated up to 9 times to induce mechanical allodynia. • Paw withdrawal threshold (PWT) used as an indicator of neuropathy • The rats showing significant mechanical allodynia ⎼ PWT 4g were used as an indicator. • TTX 8ug/kg or vehicle injected subcutaneously, q.d. for 5 days • Duloxetine given orally, at 30 mg/kg, q.d.as active control (3,750 X the TTX dose) 0 2 4 6 8 10 12 14 Pre1 Pre2 Pre3 W1 W2 D1-1h D2-1h D3-1h D4-1h D5-1h D7 D10 D14 PWT (g) Effect of TTX on 1h PWT in Oxaliplatin induced pain model rats Placebo 1 ml/kg, sc TTX 8ug/kg, sc Duloxetine 30 mg/kg, po Dosing, qd. * ** *** ** *** ** ** *,**,***: p<0.05, 0.01, 0.001, respectively, compared to placebo group, one-way ANOVA, n=7 *** *** *** Halneuron Effect on PWT in Oxaliplatin Induced Rat Pain Model |
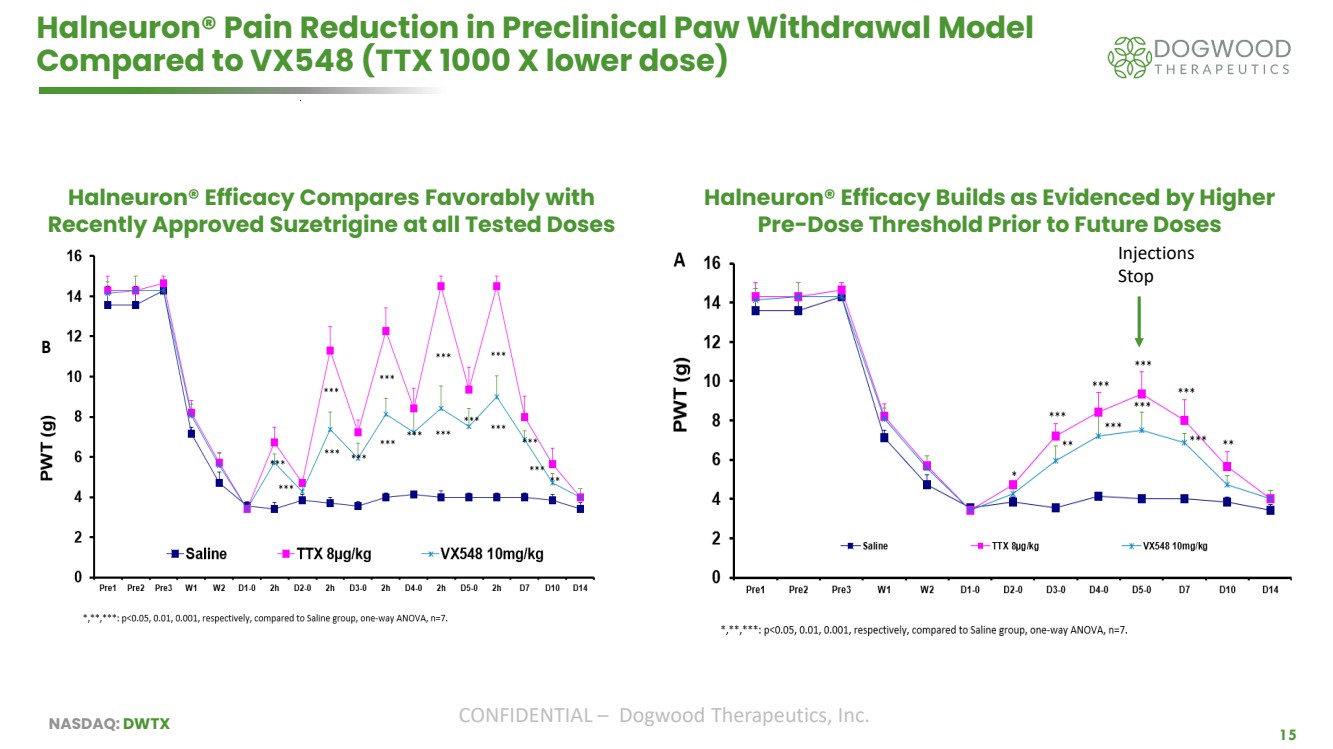
| NASDAQ: DWTX CONFIDENTIAL – Dogwood Therapeutics, Inc. 15 Halneuron® Pain Reduction in Preclinical Paw Withdrawal Model Compared to VX548 (TTX 1000 X lower dose) Halneuron® Efficacy Builds as Evidenced by Higher Pre-Dose Threshold Prior to Future Doses Halneuron® Efficacy Compares Favorably with Recently Approved Suzetrigine at all Tested Doses Injections Stop |
| NASDAQ: DWTX CONFIDENTIAL – Dogwood Therapeutics, Inc. 16 Previous Phase 2 Human Studies- Cancer Related Pain (CRP) Cancer Related Pain Phase 2 Study (n=165) • Tested for efficacy and safety of Halneuron® for moderate to severe inadequately controlled pain post cancer therapy ⎼ Included neuropathic and non-neuropathic pain patients • Randomized, double-blind, placebo-controlled, parallel-design, multicenter trial • Statistically significant efficacy achieved based on a pain reduction endpoint • On average, Halneuron® responders demonstrated pain relief for 57.7 days post injection • Halneuron® showed an acceptable safety profile in cancer patients |
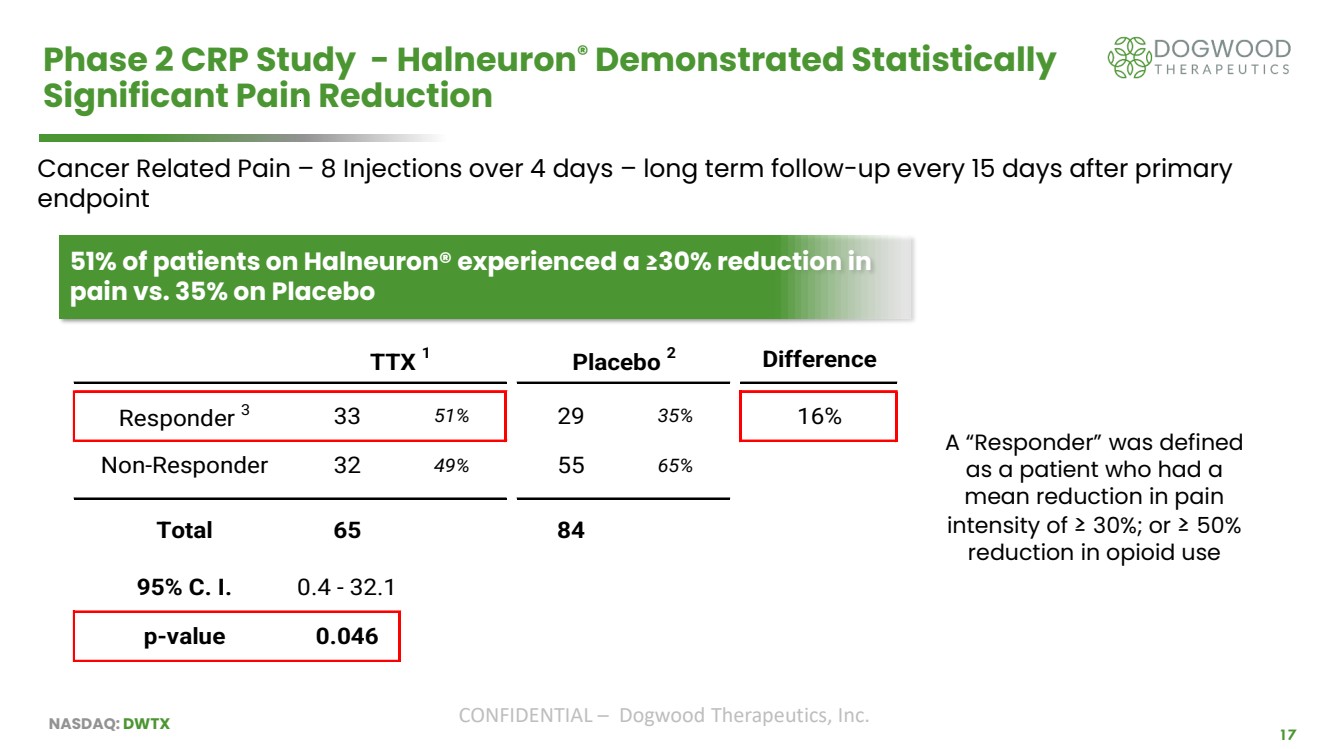
| NASDAQ: DWTX CONFIDENTIAL – Dogwood Therapeutics, Inc. 17 Phase 2 CRP Study - Halneuron® Demonstrated Statistically Significant Pain Reduction 51% of patients on Halneuron® experienced a ≥30% reduction in pain vs. 35% on Placebo TTX 1 Placebo 2 Difference Responder 3 33 51% 29 35% 16% Non-Responder 32 49% 55 65% Total 65 84 95% C. I. 0.4 - 32.1 p-value 0.046 Cancer Related Pain – 8 Injections over 4 days – long term follow-up every 15 days after primary endpoint A “Responder” was defined as a patient who had a mean reduction in pain intensity of ≥ 30%; or ≥ 50% reduction in opioid use |
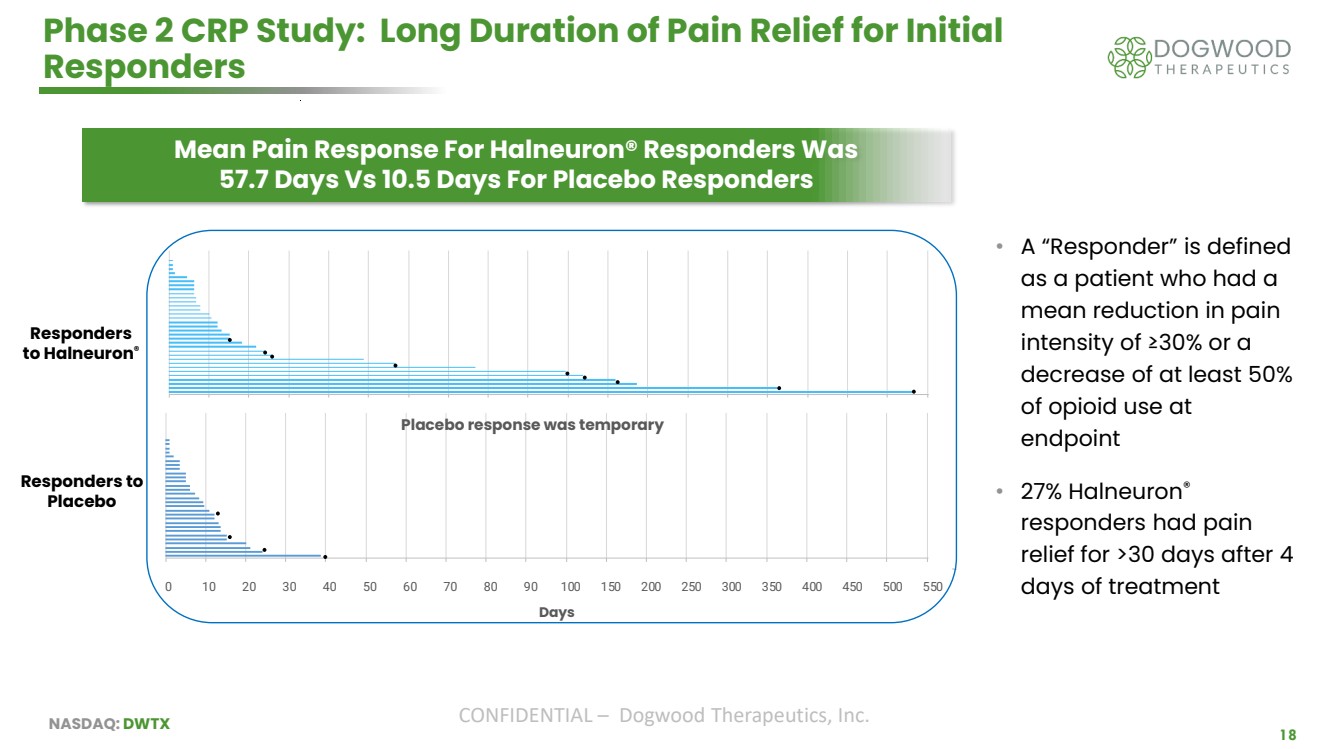
| NASDAQ: DWTX CONFIDENTIAL – Dogwood Therapeutics, Inc. 18 Phase 2 CRP Study: Long Duration of Pain Relief for Initial Responders Responders to Halneuron® Responders to Placebo Placebo response was temporary 0 10 20 30 40 50 60 70 80 90 100 150 200 250 300 350 400 450 500 550 Days • A “Responder” is defined as a patient who had a mean reduction in pain intensity of ≥30% or a decrease of at least 50% of opioid use at endpoint • 27% Halneuron® responders had pain relief for >30 days after 4 days of treatment Mean Pain Response For Halneuron® Responders Was 57.7 Days Vs 10.5 Days For Placebo Responders |
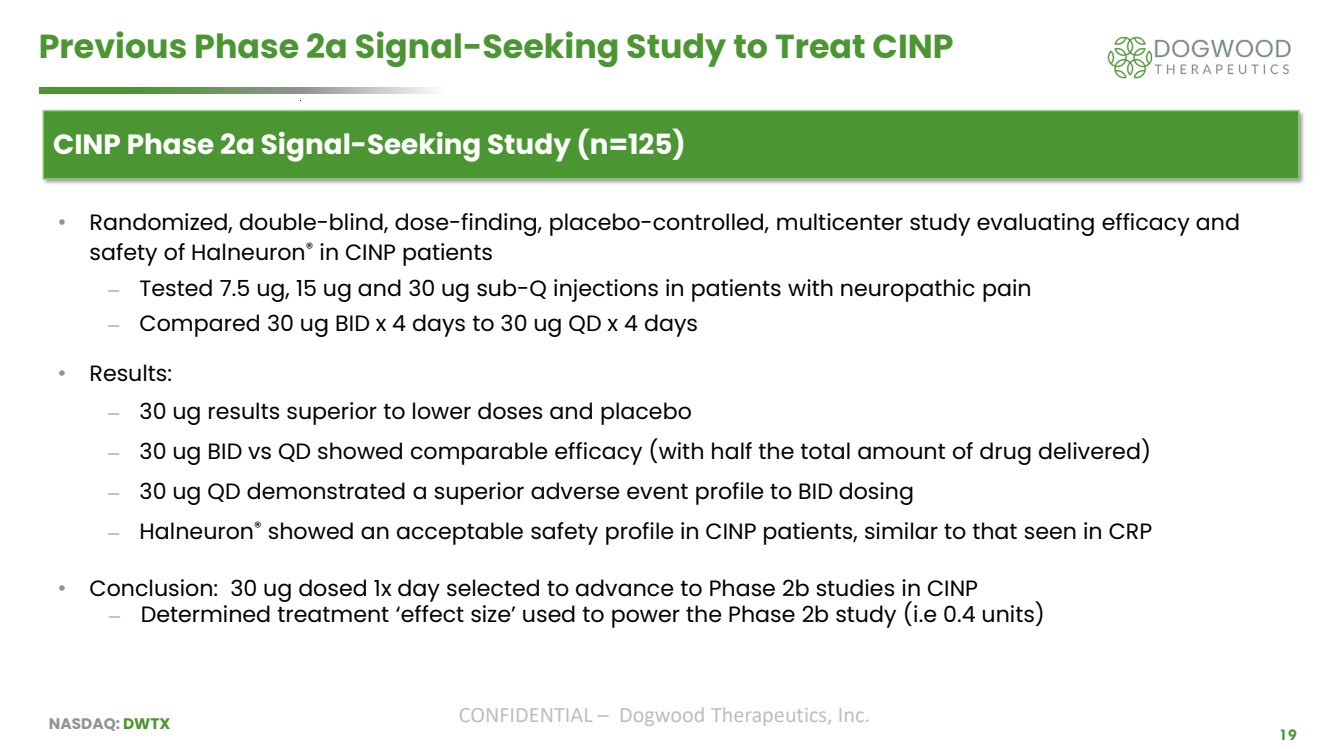
| NASDAQ: DWTX CONFIDENTIAL – Dogwood Therapeutics, Inc. 19 Previous Phase 2a Signal-Seeking Study to Treat CINP CINP Phase 2a Signal-Seeking Study (n=125) • Randomized, double-blind, dose-finding, placebo-controlled, multicenter study evaluating efficacy and safety of Halneuron® in CINP patients ⎼ Tested 7.5 ug, 15 ug and 30 ug sub-Q injections in patients with neuropathic pain ⎼ Compared 30 ug BID x 4 days to 30 ug QD x 4 days • Results: ⎼ 30 ug results superior to lower doses and placebo ⎼ 30 ug BID vs QD showed comparable efficacy (with half the total amount of drug delivered) ⎼ 30 ug QD demonstrated a superior adverse event profile to BID dosing ⎼ Halneuron® showed an acceptable safety profile in CINP patients, similar to that seen in CRP • Conclusion: 30 ug dosed 1x day selected to advance to Phase 2b studies in CINP ⎼ Determined treatment ‘effect size’ used to power the Phase 2b study (i.e 0.4 units) |
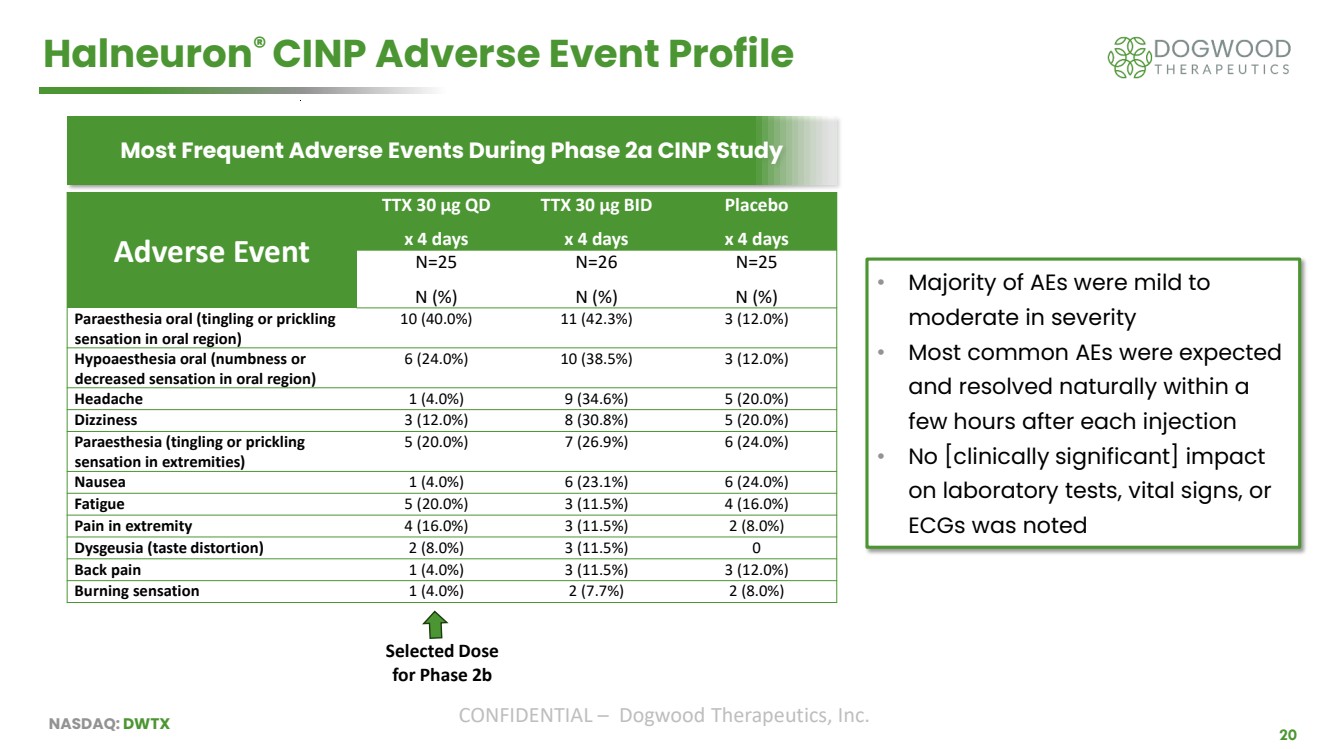
| NASDAQ: DWTX CONFIDENTIAL – Dogwood Therapeutics, Inc. 20 Halneuron® CINP Adverse Event Profile Most Frequent Adverse Events During Phase 2a CINP Study • Majority of AEs were mild to moderate in severity • Most common AEs were expected and resolved naturally within a few hours after each injection • No [clinically significant] impact on laboratory tests, vital signs, or ECGs was noted Adverse Event TTX 30 µg QD x 4 days TTX 30 µg BID x 4 days Placebo x 4 days N=25 N (%) N=26 N (%) N=25 N (%) Paraesthesia oral (tingling or prickling sensation in oral region) 10 (40.0%) 11 (42.3%) 3 (12.0%) Hypoaesthesia oral (numbness or decreased sensation in oral region) 6 (24.0%) 10 (38.5%) 3 (12.0%) Headache 1 (4.0%) 9 (34.6%) 5 (20.0%) Dizziness 3 (12.0%) 8 (30.8%) 5 (20.0%) Paraesthesia (tingling or prickling sensation in extremities) 5 (20.0%) 7 (26.9%) 6 (24.0%) Nausea 1 (4.0%) 6 (23.1%) 6 (24.0%) Fatigue 5 (20.0%) 3 (11.5%) 4 (16.0%) Pain in extremity 4 (16.0%) 3 (11.5%) 2 (8.0%) Dysgeusia (taste distortion) 2 (8.0%) 3 (11.5%) 0 Back pain 1 (4.0%) 3 (11.5%) 3 (12.0%) Burning sensation 1 (4.0%) 2 (7.7%) 2 (8.0%) Selected Dose for Phase 2b |
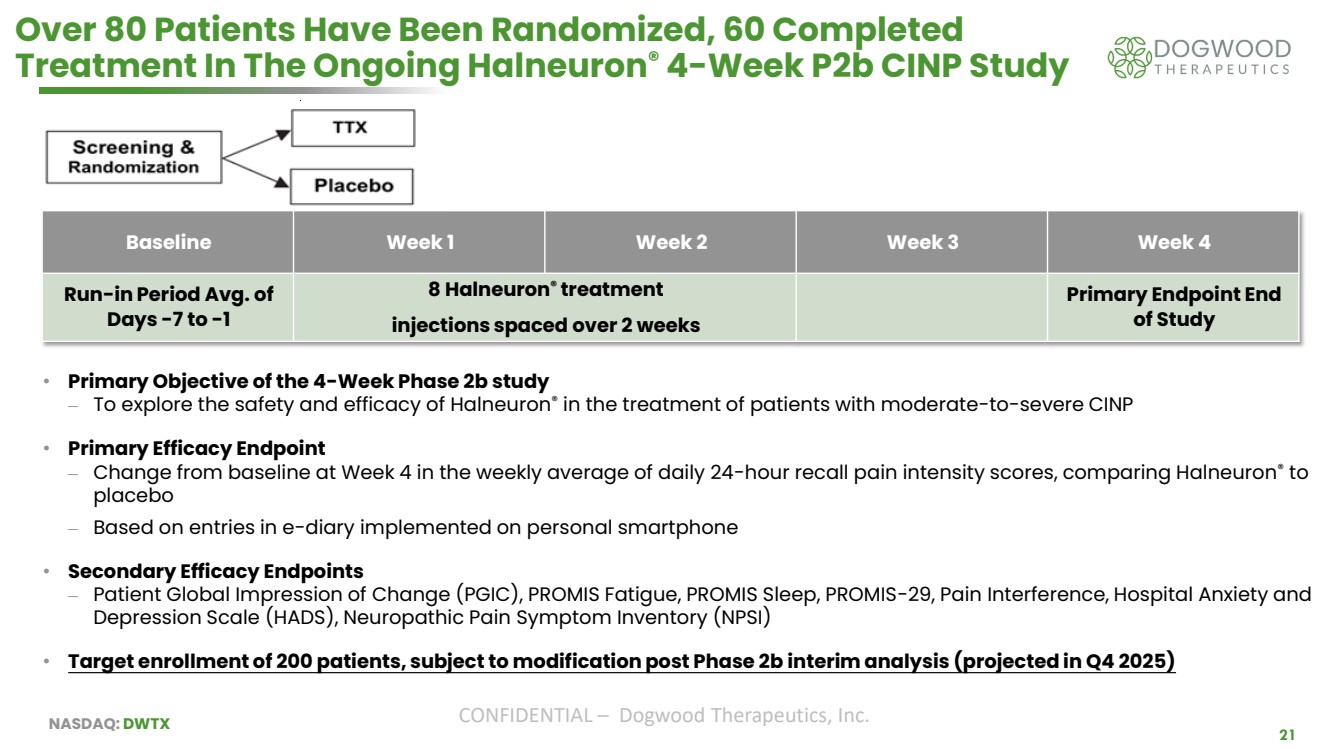
| NASDAQ: DWTX CONFIDENTIAL – Dogwood Therapeutics, Inc. 21 Over 80 Patients Have Been Randomized, 60 Completed Treatment In The Ongoing Halneuron® 4-Week P2b CINP Study Baseline Week 1 Week 2 Week 3 Week 4 Run-in Period Avg. of Days -7 to -1 8 Halneuron® treatment injections spaced over 2 weeks Primary Endpoint End of Study • Primary Objective of the 4-Week Phase 2b study ⎼ To explore the safety and efficacy of Halneuron® in the treatment of patients with moderate-to-severe CINP • Primary Efficacy Endpoint ⎼ Change from baseline at Week 4 in the weekly average of daily 24-hour recall pain intensity scores, comparing Halneuron® to placebo ⎼ Based on entries in e-diary implemented on personal smartphone • Secondary Efficacy Endpoints ⎼ Patient Global Impression of Change (PGIC), PROMIS Fatigue, PROMIS Sleep, PROMIS-29, Pain Interference, Hospital Anxiety and Depression Scale (HADS), Neuropathic Pain Symptom Inventory (NPSI) • Target enrollment of 200 patients, subject to modification post Phase 2b interim analysis (projected in Q4 2025) |
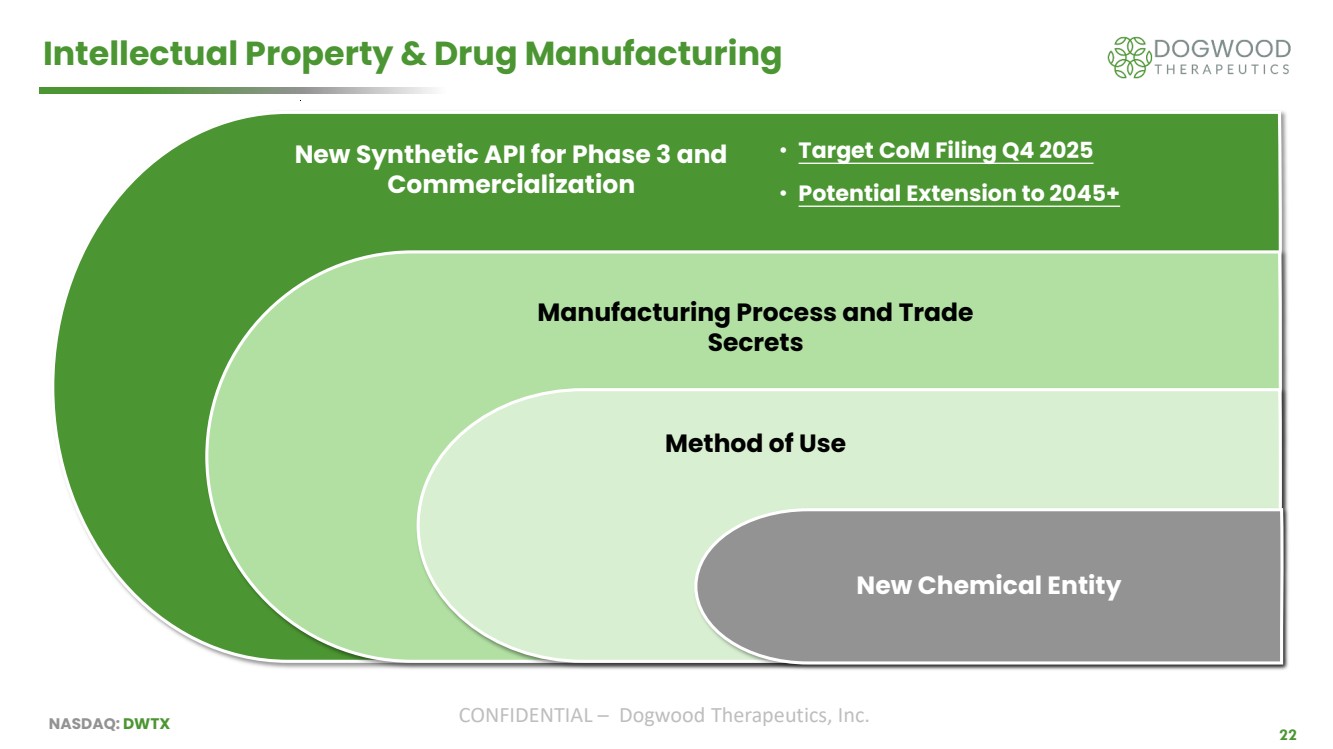
| NASDAQ: DWTX CONFIDENTIAL – Dogwood Therapeutics, Inc. 22 Intellectual Property & Drug Manufacturing New Synthetic API for Phase 3 and Commercialization Manufacturing Process and Trade Secrets Method of Use • Target CoM Filing Q4 2025 • Potential Extension to 2045+ New Chemical Entity |
| CONFIDENTIAL – Dogwood Therapeutics, Inc. SP16 IV CIPN Program Overview |
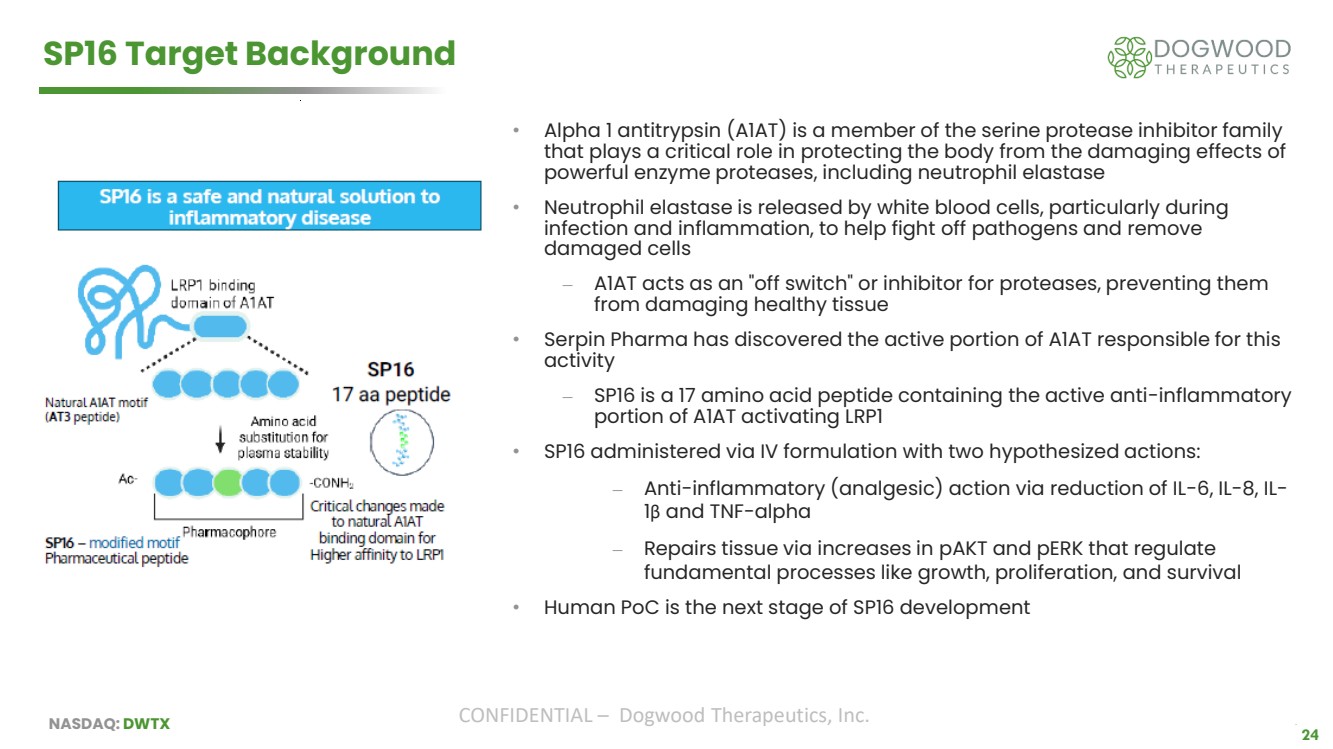
| NASDAQ: DWTX CONFIDENTIAL – Dogwood Therapeutics, Inc. 24 SP16 Target Background • Alpha 1 antitrypsin (A1AT) is a member of the serine protease inhibitor family that plays a critical role in protecting the body from the damaging effects of powerful enzyme proteases, including neutrophil elastase • Neutrophil elastase is released by white blood cells, particularly during infection and inflammation, to help fight off pathogens and remove damaged cells ⎼ A1AT acts as an "off switch" or inhibitor for proteases, preventing them from damaging healthy tissue • Serpin Pharma has discovered the active portion of A1AT responsible for this activity ⎼ SP16 is a 17 amino acid peptide containing the active anti-inflammatory portion of A1AT activating LRP1 • SP16 administered via IV formulation with two hypothesized actions: ⎼ Anti-inflammatory (analgesic) action via reduction of IL-6, IL-8, IL-1β and TNF-alpha ⎼ Repairs tissue via increases in pAKT and pERK that regulate fundamental processes like growth, proliferation, and survival • Human PoC is the next stage of SP16 development 24 |
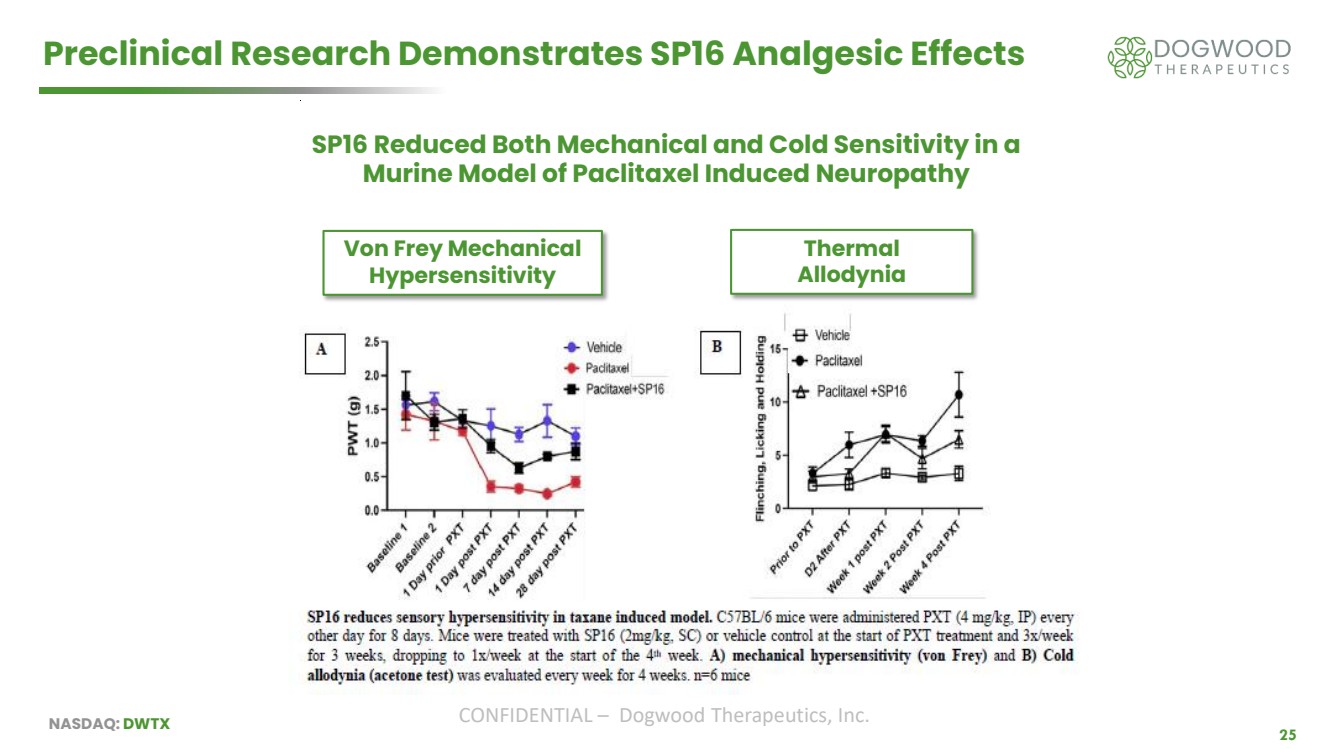
| NASDAQ: DWTX CONFIDENTIAL – Dogwood Therapeutics, Inc. 25 Preclinical Research Demonstrates SP16 Analgesic Effects Von Frey Mechanical Hypersensitivity Thermal Allodynia SP16 Reduced Both Mechanical and Cold Sensitivity in a Murine Model of Paclitaxel Induced Neuropathy |
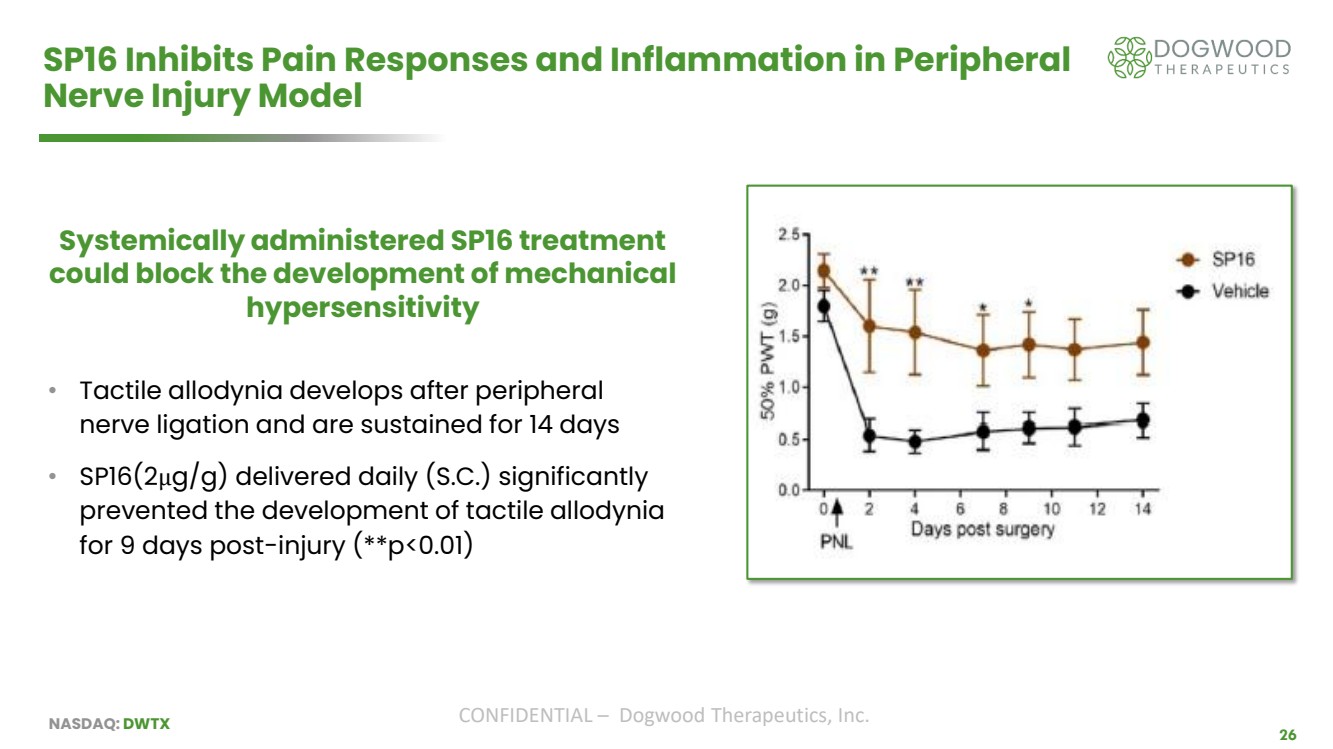
| NASDAQ: DWTX CONFIDENTIAL – Dogwood Therapeutics, Inc. 26 SP16 Inhibits Pain Responses and Inflammation in Peripheral Nerve Injury Model Systemically administered SP16 treatment could block the development of mechanical hypersensitivity • Tactile allodynia develops after peripheral nerve ligation and are sustained for 14 days • SP16(2μg/g) delivered daily (S.C.) significantly prevented the development of tactile allodynia for 9 days post-injury (**p<0.01) |
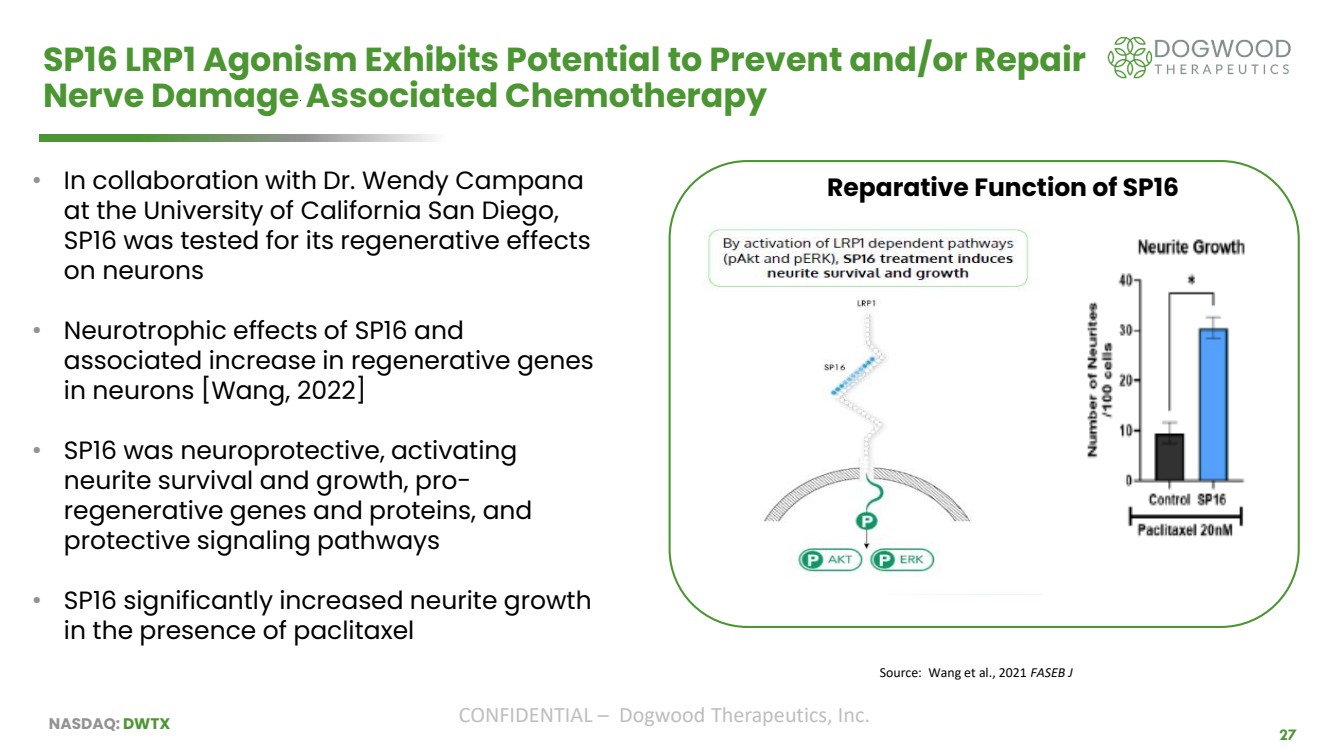
| NASDAQ: DWTX CONFIDENTIAL – Dogwood Therapeutics, Inc. 27 SP16 LRP1 Agonism Exhibits Potential to Prevent and/or Repair Nerve Damage Associated Chemotherapy • In collaboration with Dr. Wendy Campana at the University of California San Diego, SP16 was tested for its regenerative effects on neurons • Neurotrophic effects of SP16 and associated increase in regenerative genes in neurons [Wang, 2022] • SP16 was neuroprotective, activating neurite survival and growth, pro-regenerative genes and proteins, and protective signaling pathways • SP16 significantly increased neurite growth in the presence of paclitaxel Source: Wang et al., 2021 FASEB J Reparative Function of SP16 |
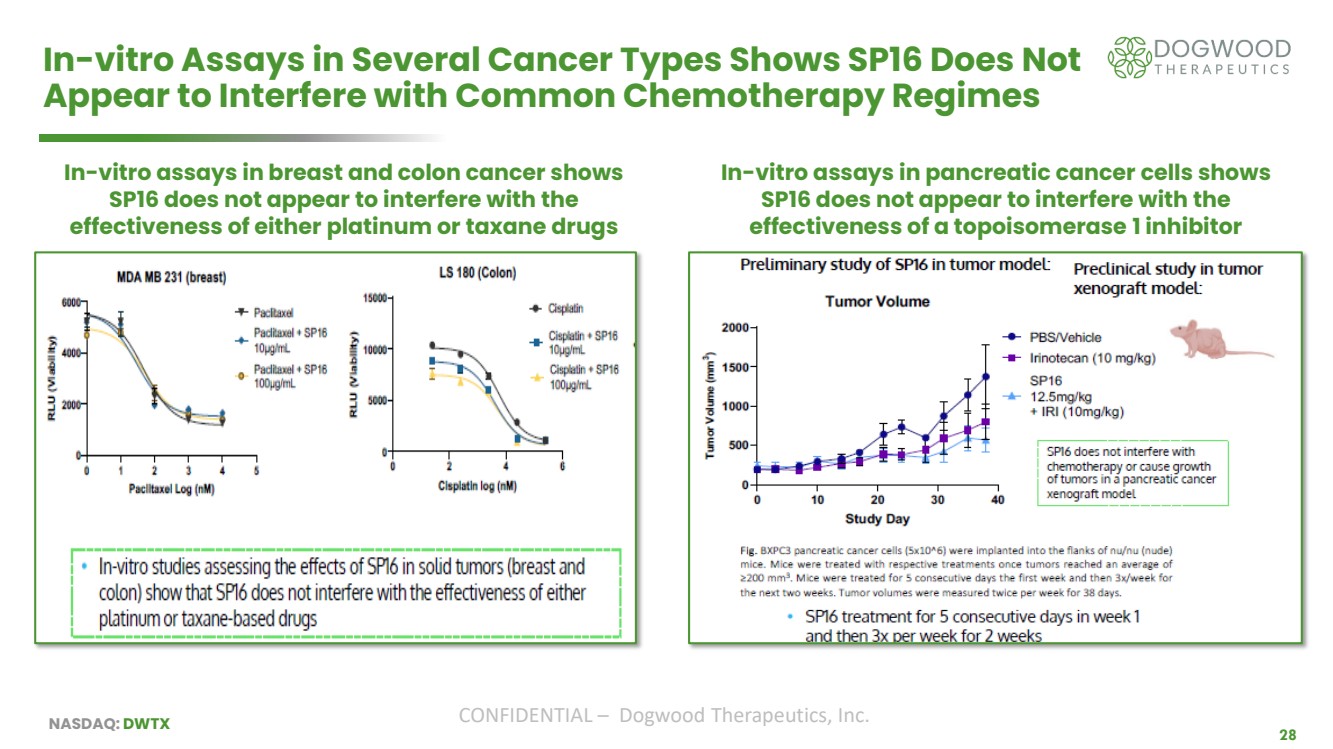
| NASDAQ: DWTX CONFIDENTIAL – Dogwood Therapeutics, Inc. 28 In-vitro Assays in Several Cancer Types Shows SP16 Does Not Appear to Interfere with Common Chemotherapy Regimes In-vitro assays in breast and colon cancer shows SP16 does not appear to interfere with the effectiveness of either platinum or taxane drugs In-vitro assays in pancreatic cancer cells shows SP16 does not appear to interfere with the effectiveness of a topoisomerase 1 inhibitor |
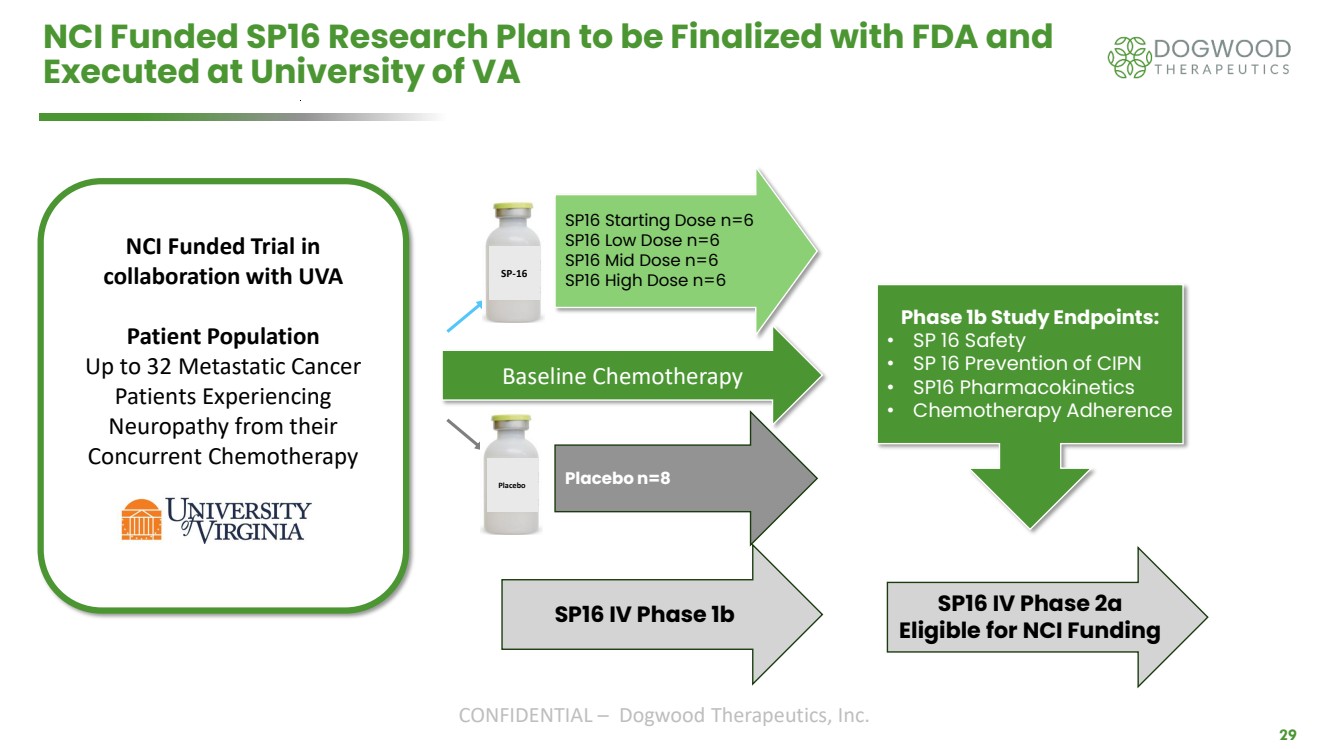
| CONFIDENTIAL – Dogwood Therapeutics, Inc. 29 NCI Funded SP16 Research Plan to be Finalized with FDA and Executed at University of VA Baseline Chemotherapy SP16 Starting Dose n=6 SP16 Low Dose n=6 SP16 Mid Dose n=6 SP16 High Dose n=6 Placebo n=8 SP16 IV Phase 1b SP16 IV Phase 2a Eligible for NCI Funding Phase 1b Study Endpoints: • SP 16 Safety • SP 16 Prevention of CIPN • SP16 Pharmacokinetics • Chemotherapy Adherence NCI Funded Trial in collaboration with UVA Patient Population Up to 32 Metastatic Cancer Patients Experiencing Neuropathy from their Concurrent Chemotherapy SP-16 Placebo |
| NASDAQ: DWTX CONFIDENTIAL – Dogwood Therapeutics, Inc. 30 Corporate Summary • Halneuron® (TTX) = Nav1.7 channel blocker, analgesic → potentially best for treating established CINP pain ⎼ Halneuron® is in Phase 2b development for CINP ⎼ Opportunity to expand ⎼ Nav1.7 channel blocker should have utility on cancer related pain, as well as post surgical pain • SP16 = LRP1-agonist, anti-inflammatory, neuroprotection → potentially best for attenuation of CIPN during chemo; ⎼ SP16 is in early clinical stage development and may enable neuroprotection, may preserve full chemo regimen and potential to synergistically complement Halneuron® in treating pain post chemotherapy ⎼ SP16 Phase 1b development for CIPN ⎼ Program is fully funded by NCI through next clinical milestone • Common commercial call points (oncology and pain Centers) and potential partners • Bundled protocols/formularies with major cancer centers/providers ⎼ Co-promotion targeting infusion suites and pain clinics • Together deeper penetration into the global CINP treatment opportunity ~$1.5B market ⎼ Ability to expand into larger ~$5B cancer pain market |
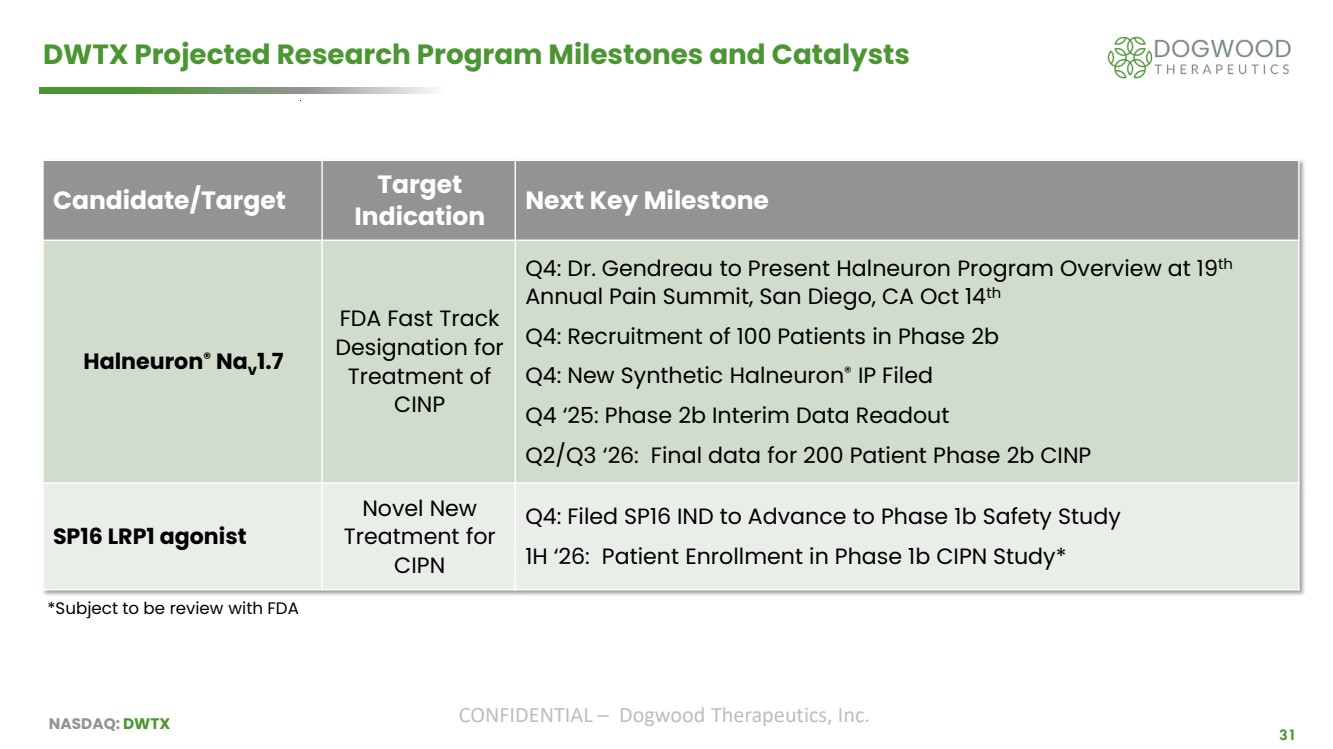
| NASDAQ: DWTX CONFIDENTIAL – Dogwood Therapeutics, Inc. 31 DWTX Projected Research Program Milestones and Catalysts Candidate/Target Target Indication Next Key Milestone Halneuron® Nav 1.7 FDA Fast Track Designation for Treatment of CINP Q4: Dr. Gendreau to Present Halneuron Program Overview at 19th Annual Pain Summit, San Diego, CA Oct 14th Q4: Recruitment of 100 Patients in Phase 2b Q4: New Synthetic Halneuron® IP Filed Q4 ‘25: Phase 2b Interim Data Readout Q2/Q3 ‘26: Final data for 200 Patient Phase 2b CINP SP16 LRP1 agonist Novel New Treatment for CIPN Q4: Filed SP16 IND to Advance to Phase 1b Safety Study 1H ‘26: Patient Enrollment in Phase 1b CIPN Study* *Subject to be review with FDA |
| CONFIDENTIAL – Dogwood Therapeutics, Inc. Investor Relations Email: IR@dwtx.com NASDAQ: DWTX |