

New Scientific Paradigm Exploring Herpes Virus Activation as Potential Underlying Cause of Fibromyalgia, Long COVID and Other Chronic Conditions Nasdaq: VIRI September 2022 Filed Pursuant to Rule 433 Issuer Free Writing Prospectus dated September 19, 2022 File No. 333 - 263700

NASDAQ: VIRI Forward Looking Statements 2 • Statements in this presentation contain “forward - looking statements” that are subject to substantial risks and uncertainties. Fo rward - looking statements contained in this presentation may be identified by the use of words such as “anticipate,” “expect,” “believe,” “will,” “may,” “should,” “estimate,” “project,” “ou tlo ok,” “forecast” or other similar words, and include, without limitation, all statements other than those regarding historical facts, statements regarding Virios Therapeutics, Inc.’s expectations regardi ng our future financial or business performance, plans, prospects, trends or strategies, objectives of management, competition and other financial and business matters; the potential, safety, eff icacy, and regulatory and clinical progress of our current and prospective product candidates, planned clinical trials and preclinical activities, and projected research and development costs ; the estimated size of the market for our product candidates; and the timing and success of our development and commercialization of our anticipated product candidates and the market acceptance thereof. Forward - looking statements are based on our current expectations and are subject to inherent uncertainties, risks and assumptions that are difficult to predict. Further, ce rtain forward - looking statements are based on assumptions as to future events that may not prove to be accurate. These statements are neither promises nor guarantees, but involve known and unk nown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or ach ievements expressed or implied by the forward - looking statements, including, but not limited to, the following: the ongoing effects of COVID - 19 has adversely impacted and may continue to adversely impact our business, including our preclinical studies and clinical trials; our limited operating history, which may make it difficult to evaluate our current business and pre dict our future success and viability; we have and expect to continue to incur significant losses; our need for additional funding, which may not be available; our substantial dependence on the succ ess of our lead product candidates; failure to identify additional product candidates and develop or commercialize marketable products; the early stage of our development efforts; potential un for eseen events during clinical trials could cause delays or other adverse consequences; risks relating to the regulatory approval process or ongoing regulatory obligations; our product candid ate s may cause serious adverse side effects; our reliance on third parties; effects of significant competition; the possibility of system failures or security breaches; risks relating to intel lec tual property; our ability to attract, retain and motivate qualified personnel; and significant costs as a result of operating as a public company. These and other risks and uncertainties are de scr ibed more fully in the section titled “Risk Factors” in the Annual Report on Form 10 - K for the year ended December 31, 2021 filed with the Securities and Exchange Commission (“SEC”) and elsewhere in our filings and reports with the SEC. While we may elect to update these forward - looking statements at some point in the future, we assume no obligation to update or revise any fo rward - looking statements except to the extent required by applicable law. Although we believe the expectations reflected in such forward - looking statements are reasonable, we can give no assurance that such expectations will prove to be correct. Accordingly, readers are cautioned not to place undue reliance on these forward - looking statements. No representations or warran ties (expressed or implied) are made about the accuracy of any such forward - looking statements. • This presentation also contains estimates and other statistical data made by independent parties and by us relating to market si ze and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such data and estimates. In addition, p rojections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncerta int y and risk. Neither we nor our affiliates, advisors or representatives makes any representation as to the accuracy or completeness of that data or undertake to update such data aft er the date of this presentation. • You should read the documents that we have filed with the SEC for more complete information about us. We encourage you to rea d s uch documents in full for more detailed information on statistics, reports and clinical trials referenced in this presentation. You may access these documents for free by visiting ED GAR on the SEC website at http://www.sec.gov.

NASDAQ: VIRI Free Writing Prospectus 3 • This presentation highlights basic information about us and the proposed offering. Because it is a summary, it does not conta in all of the information that you should consider before investing. We have filed a registration statement (including a preliminary prospectus supplement and the accompanying prospectus) with t he SEC for the offering to which this presentation relates. Before you invest, you should read the prospectus supplement and the accompanying prospectus in the registration statement (i ncl uding the risk factors described therein) and other documents we have filed with the SEC for more complete information about us and the offering. • You may access these documents for free by visiting EDGAR on the SEC Web site at http://www.sec.gov. The preliminary prospect us supplement is available on the SEC Web site at http://www.sec.gov. Alternatively, we or any underwriter participating in the offering will arrange to send you the prospectu s i f you contact ThinkEquity , Prospectus Department, 17 State Street, 41st Floor, New York, New York 10004, telephone: (877) 436 - 3673 or e - mail: prospectus@think - equity.com. • This presentation shall not constitute an offer to sell, or the solicitation of an offer to buy, nor will there be any sale o f t hese securities in any state or other jurisdiction in which such offer, solicitation or sale would be unlawful prior to the registration or qualification under the securities laws of such state or jur isdiction. The offering will only be made by means of a prospectus supplement and related base prospectus.

NASDAQ: VIRI Proven Leadership Team with Extensive Experience in Drug Development and Commercialization 4 Pharma Brand Development & Commercialization Experience Includes Management of: Greg Duncan Chairman & CEO R. Michael Gendreau MD, PhD CMO Ralph Grosswald SVP of Operations Angela Walsh SVP of Finance EXECUTIVE TEAM DIRECTORS Rich Whitley, MD • Distinguished Professor, UAB • Remdesivir was Originally Developed by Dr. Whitley’s team at UAB • DSMB Chair, Operation Warp Speed Abel De La Rosa, PhD • Chairman, Co - Founder Anitos Therapeutics • Led Bus Dev for Pharmasset acquisition by GILD for $11.5 billion in 2012 • Leadership for Development Programs for the Treatment of HIV, Hepatitis B & C, including Sofosbuvir John Thomas, CPA • CorMatrix Inc., MiMedx Group, Inc., DARA BioSciences , GMP Companies • MRI Interventions, EnterMed , Inc., • Medicis Pharm Corp., CytRx Corp Rick Keefer • 30 - year Pharma industry veteran with broad - based experience in leading commercial operations • Executive roles at Pharmacia, Pfizer, Wyeth, Biovail and Publicis Health • Seven - time winner of Pharma Voice’s top 100 healthcare leaders Rick Burch • 30 years at PFE including SVP • VP and GM UCB Pharmaceuticals • Former President of VIRI, Inc. • Product launches include Lyrica & Celebrex Skip Pridgen, MD VIRI Founder • Company Founder • Board - certified surgeon practicing with Tuscaloosa Surgical Associates, P.C. • Served as a physician and surgeon in the U.S. Navy
NASDAQ: VIRI Fibromyalgia Syndrome Overview 5 • American College of Rheumatology Estimates 2 - 4% of Population has FM • Hallmark Characteristics are Widespread Chronic Pain and Severe Fatigue • Symptoms Present for ≥ 3 Months • Other Symptoms May Include GI, Sleep, Mood Disorder and Headache • Higher prevalence in females: 70% FM Characteristics Sources: The Hidden Impact of Musculoskeletal Disorders on Americans, 4 th edition; Berger et al Clin Pract 2007; White et al J Occup Environ Med 2008; Wolfe et al Arthritis Care & Res 2014; Fitzcharles et al Am J Med 2011; Robinson et al Pain Medicine 2012; Peng et al Clin J Pain 2015, Chad S Boomershine , MD, PhD, CPI, CPT, Medscape , 2022; Verified Market Research , FM Report 2021 • Patients with FM > 3x Risk of Committing Suicide v. General Population • High Healthcare Utilization • Avg 10 Office Visits/Year • Significant Disability • One in two patients miss work • Estimates Suggest as Many as 40% of FM Patients are Treated with Opioids Devastating Impact
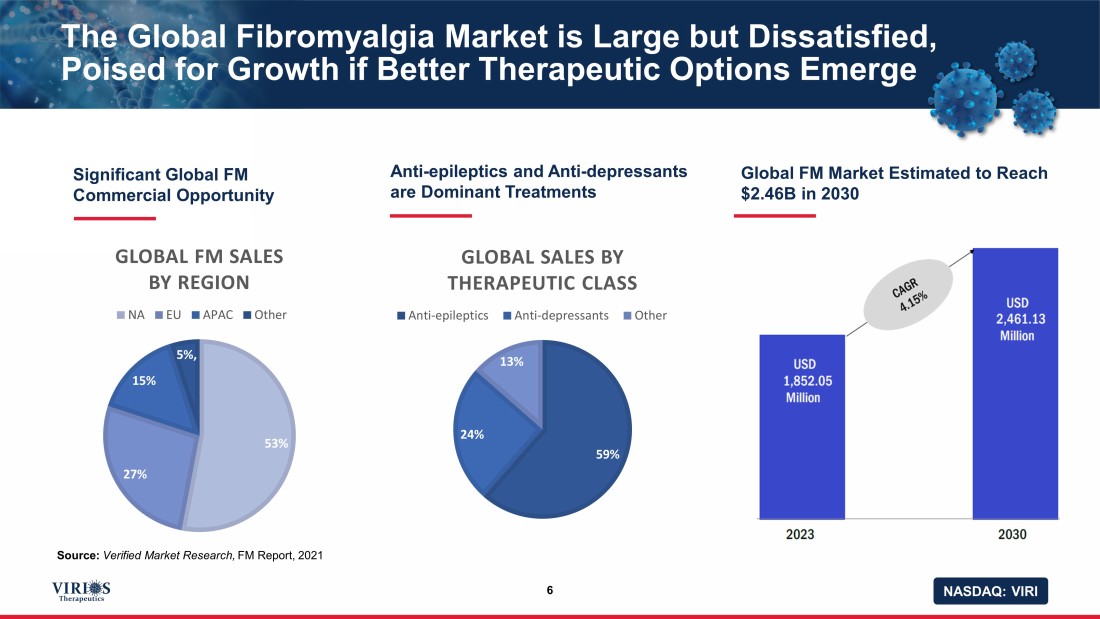
NASDAQ: VIRI The Global Fibromyalgia Market is Large but Dissatisfied, Poised for Growth if Better Therapeutic Options Emerge 6 Significant Global FM Commercial Opportunity Source: Verified Market Research, FM Report, 2021 Anti - epileptics and Anti - depressants are Dominant Treatments Global FM Market Estimated to Reach $2.46B in 2030 75% 25% 59% 24% 13% GLOBAL SALES BY THERAPEUTIC CLASS Anti-epileptics Anti-depressants Other 53% 27% 15% 5% , GLOBAL FM SALES BY REGION NA EU APAC Other
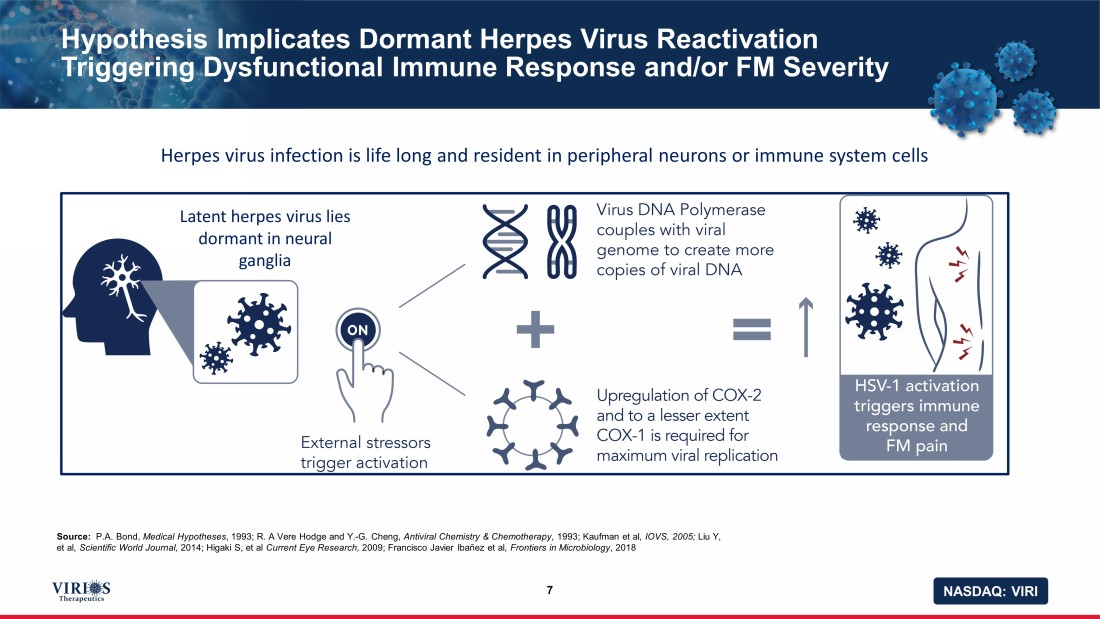
NASDAQ: VIRI Hypothesis Implicates Dormant Herpes Virus Reactivation Triggering Dysfunctional Immune Response and/or FM Severity 7 Source: P.A. Bond, Medical Hypotheses , 1993; R. A Vere Hodge and Y. - G. Cheng, Antiviral Chemistry & Chemotherapy , 1993; Kaufman et al, IOVS, 2005; Liu Y, et al, Scientific World Journal, 2014; Higaki S, et al Current Eye Research, 2009; Francisco Javier Ibañez et al, Frontiers in Microbiology , 2018 Latent herpes virus lies dormant in neural ganglia Herpes virus infection is life long and resident in peripheral neurons or immune system cells
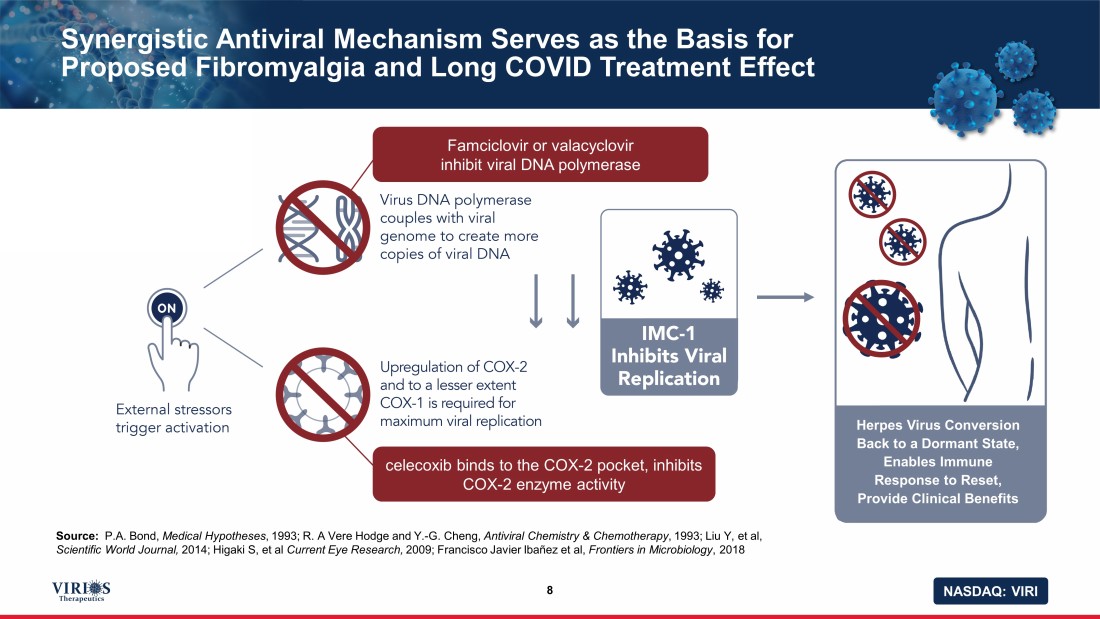
NASDAQ: VIRI Synergistic Antiviral Mechanism Serves as the Basis for Proposed Fibromyalgia and Long COVID Treatment Effect 8 Source: P.A. Bond, Medical Hypotheses , 1993; R. A Vere Hodge and Y. - G. Cheng, Antiviral Chemistry & Chemotherapy , 1993; Liu Y, et al, Scientific World Journal, 2014; Higaki S, et al Current Eye Research, 2009; Francisco Javier Ibañez et al, Frontiers in Microbiology , 2018 Famciclovir or valacyclovir inhibit viral DNA polymerase celecoxib binds to the COX - 2 pocket, inhibits COX - 2 enzyme activity Herpes Virus Conversion Back to a Dormant State, Enables Immune Response to Reset, Provide Clinical Benefits
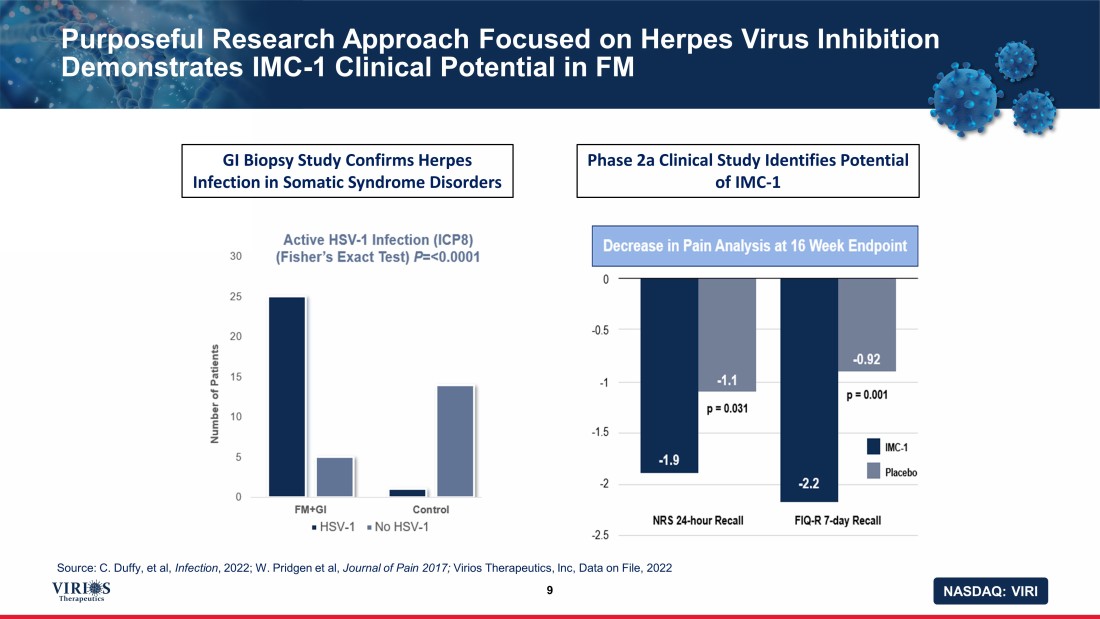
NASDAQ: VIRI Purposeful Research Approach Focused on Herpes Virus Inhibition Demonstrates IMC - 1 Clinical Potential in FM 9 GI Biopsy Study Confirms Herpes Infection in Somatic Syndrome Disorders Phase 2a Clinical Study Identifies Potential of IMC - 1 Source: C. Duffy, et al, Infection , 2022; W. Pridgen et al, Journal of Pain 2017; Virios Therapeutics, Inc, Data on File, 2022
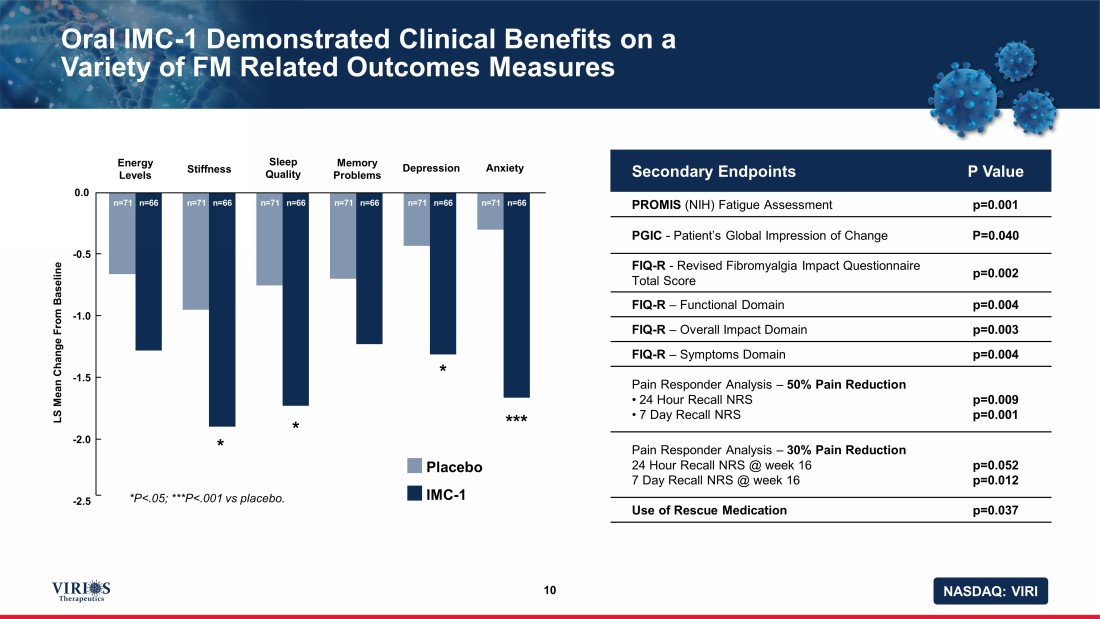
NASDAQ: VIRI Oral IMC - 1 Demonstrated Clinical Benefits on a Variety of FM Related Outcomes Measures 10 Secondary Endpoints P Value PROMIS (NIH) Fatigue Assessment p=0.001 PGIC - Patient’s Global Impression of Change P=0.040 FIQ - R - Revised Fibromyalgia Impact Questionnaire Total Score p=0.002 FIQ - R – Functional Domain p=0.004 FIQ - R – Overall Impact Domain p=0.003 FIQ - R – Symptoms Domain p=0.004 Pain Responder Analysis – 50% Pain Reduction • 24 Hour Recall NRS • 7 Day Recall NRS p=0.009 p=0.001 Pain Responder Analysis – 30% Pain Reduction 24 Hour Recall NRS @ week 16 7 Day Recall NRS @ week 16 p=0.052 p=0.012 Use of Rescue Medication p=0.037 n=71 n=66 n=71 n=66 n=71 n=66 n=71 n=66 n=71 n=66 n=71 n=66 Energy Levels LS Mean Change From Baseline Stiffness Sleep Quality Memory Problems Depression Anxiety 0.0 - 0.5 - 1.0 - 1.5 - 2.0 - 2.5 *P<.05; ***P<.001 vs placebo. * * * *** Placebo IMC - 1

NASDAQ: VIRI FORTRESS Clinical Trial Design 11 • 425 Female Patients Enrolled 18 - 65 Years of Age, 422 ITT population • 1:1 IMC - 1 (675mg famciclovir + 180mg celecoxib) vs Placebo, Dosed BID • Double - blind, 41 US Research Centers • Diagnosis of Fibromyalgia Using 2016 ACR Criteria Design Summary: Primary Endpoints: Reduction in Pain Key Secondary Endpoints: PGIC, FIQ - R Domains, 30% & 50% pain responder analyses 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 14 weeks of IMC - 1 or Placebo Treatment, Followed by Two Week Placebo Washout for All Subjects IMC - 1 Placebo Prospectively Defined Primary Endpoint Analysis
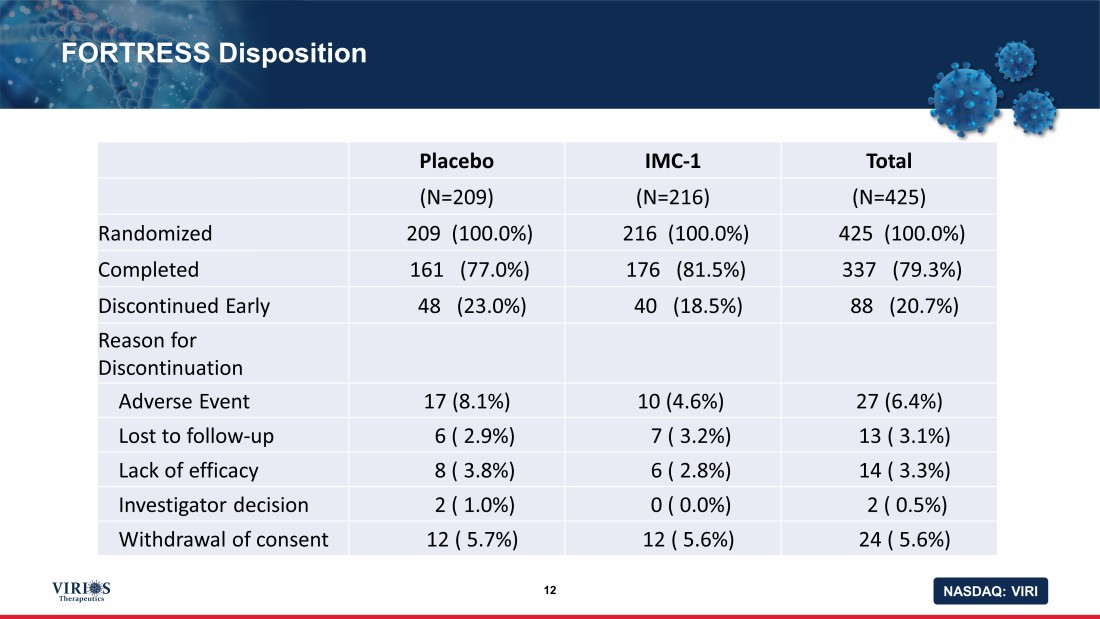
NASDAQ: VIRI FORTRESS Disposition 12 Placebo IMC - 1 Total (N=209) (N=216) (N=425) Randomized 209 (100.0%) 216 (100.0%) 425 (100.0%) Completed 161 (77.0%) 176 (81.5%) 337 (79.3%) Discontinued Early 48 (23.0%) 40 (18.5%) 88 (20.7%) Reason for Discontinuation Adverse Event 17 (8.1%) 10 (4.6%) 27 (6.4%) Lost to follow - up 6 ( 2.9%) 7 ( 3.2%) 13 ( 3.1%) Lack of efficacy 8 ( 3.8%) 6 ( 2.8%) 14 ( 3.3%) Investigator decision 2 ( 1.0%) 0 ( 0.0%) 2 ( 0.5%) Withdrawal of consent 12 ( 5.7%) 12 ( 5.6%) 24 ( 5.6%)
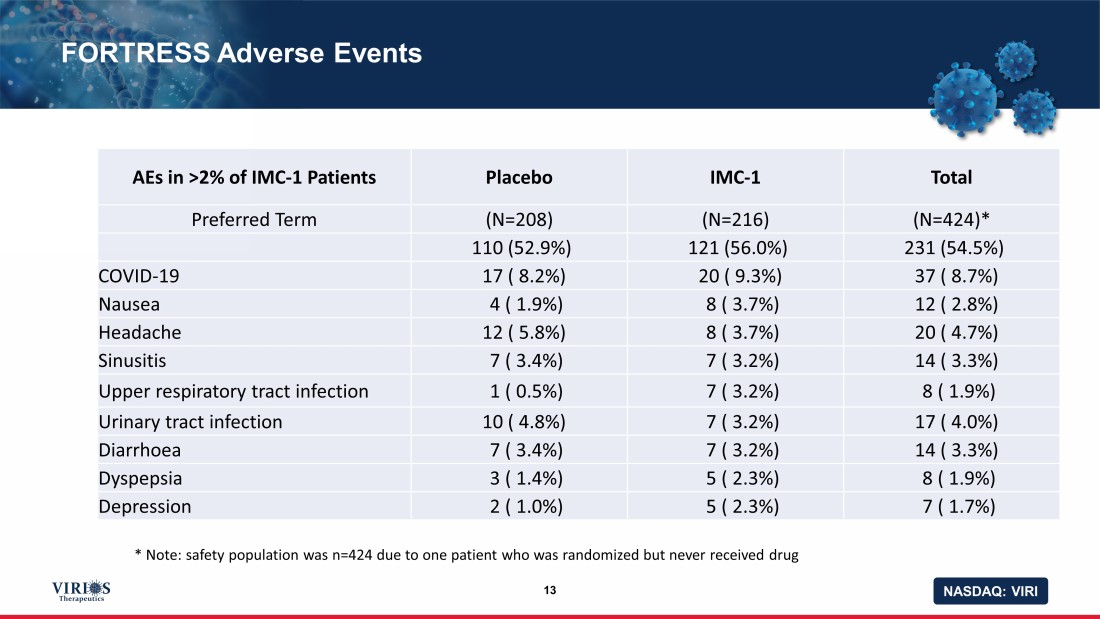
NASDAQ: VIRI FORTRESS Adverse Events 13 AEs in >2% of IMC - 1 Patients Placebo IMC - 1 Total Preferred Term (N=208) (N=216) (N=424)* 110 (52.9%) 121 (56.0%) 231 (54.5%) COVID - 19 17 ( 8.2%) 20 ( 9.3%) 37 ( 8.7%) Nausea 4 ( 1.9%) 8 ( 3.7%) 12 ( 2.8%) Headache 12 ( 5.8%) 8 ( 3.7%) 20 ( 4.7%) Sinusitis 7 ( 3.4%) 7 ( 3.2%) 14 ( 3.3%) Upper respiratory tract infection 1 ( 0.5%) 7 ( 3.2%) 8 ( 1.9%) Urinary tract infection 10 ( 4.8%) 7 ( 3.2%) 17 ( 4.0%) Diarrhoea 7 ( 3.4%) 7 ( 3.2%) 14 ( 3.3%) Dyspepsia 3 ( 1.4%) 5 ( 2.3%) 8 ( 1.9%) Depression 2 ( 1.0%) 5 ( 2.3%) 7 ( 1.7%) * Note: safety population was n=424 due to one patient who was randomized but never received drug
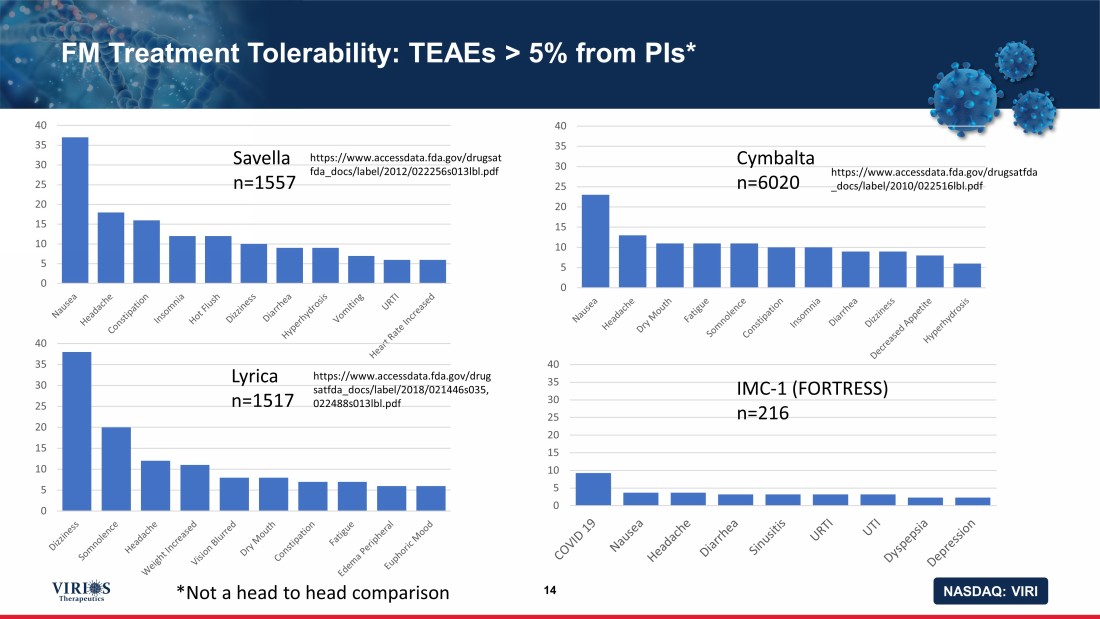
NASDAQ: VIRI FM Treatment Tolerability: TEAEs > 5% from PIs* 14 0 5 10 15 20 25 30 35 40 0 5 10 15 20 25 30 35 40 0 5 10 15 20 25 30 35 40 0 5 10 15 20 25 30 35 40 Savella n=1557 Cymbalta n=6020 Lyrica n=1517 IMC - 1 (FORTRESS) n=216 https://www.accessdata.fda.gov/drugsat fda_docs/label/2012/022256s013lbl.pdf https://www.accessdata.fda.gov/drugsatfda _docs/label/2010/022516lbl.pdf https://www.accessdata.fda.gov/drug satfda_docs/label/2018/021446s035, 022488s013lbl.pdf *Not a head to head comparison
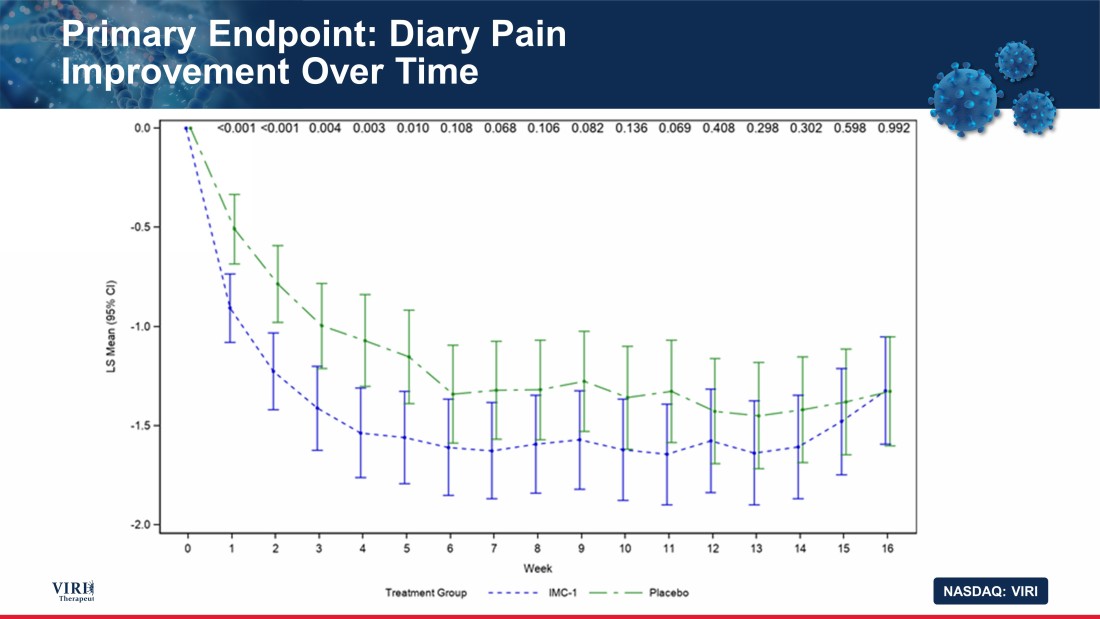
NASDAQ: VIRI Primary Endpoint: Diary Pain Improvement Over Time 15
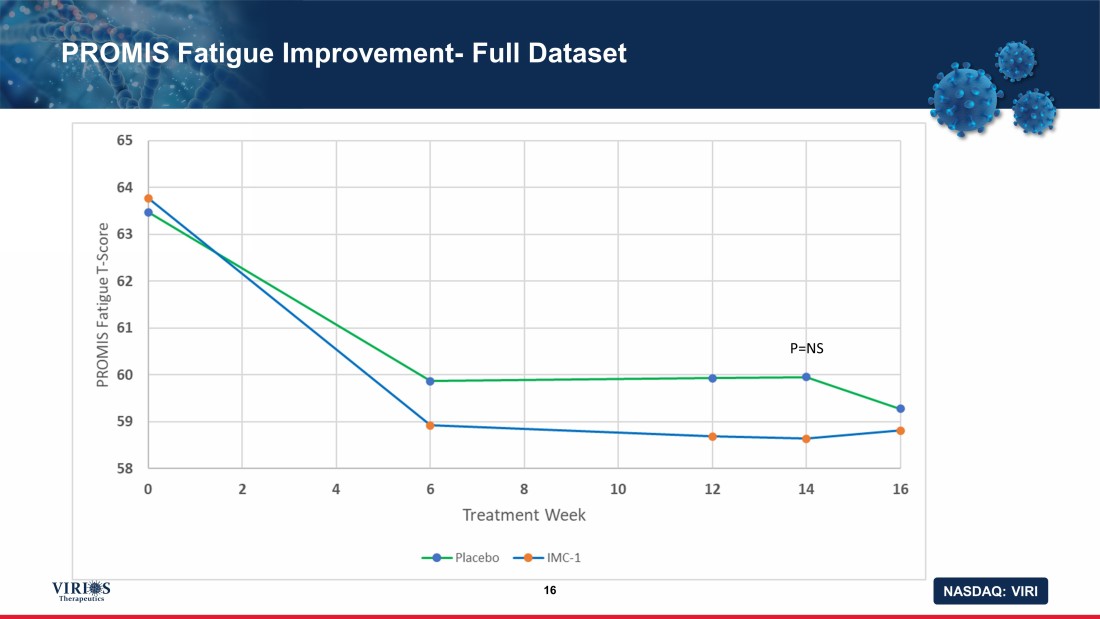
NASDAQ: VIRI PROMIS Fatigue Improvement - Full Dataset 16 P=NS
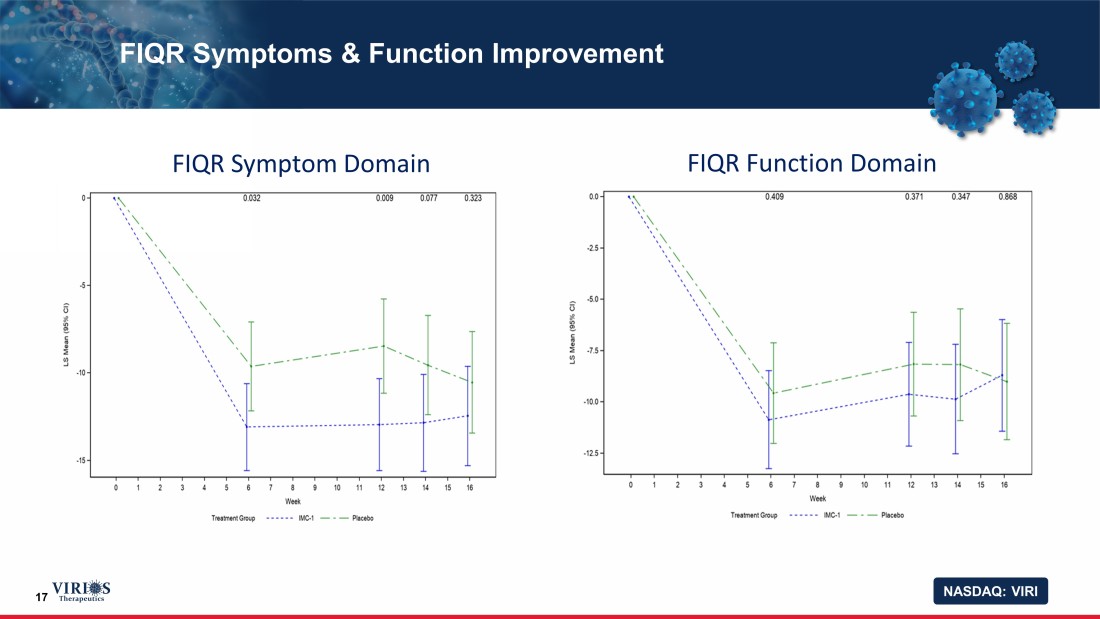
NASDAQ: VIRI 17 FIQR Symptoms & Function Improvement FIQR Symptom Domain FIQR Function Domain
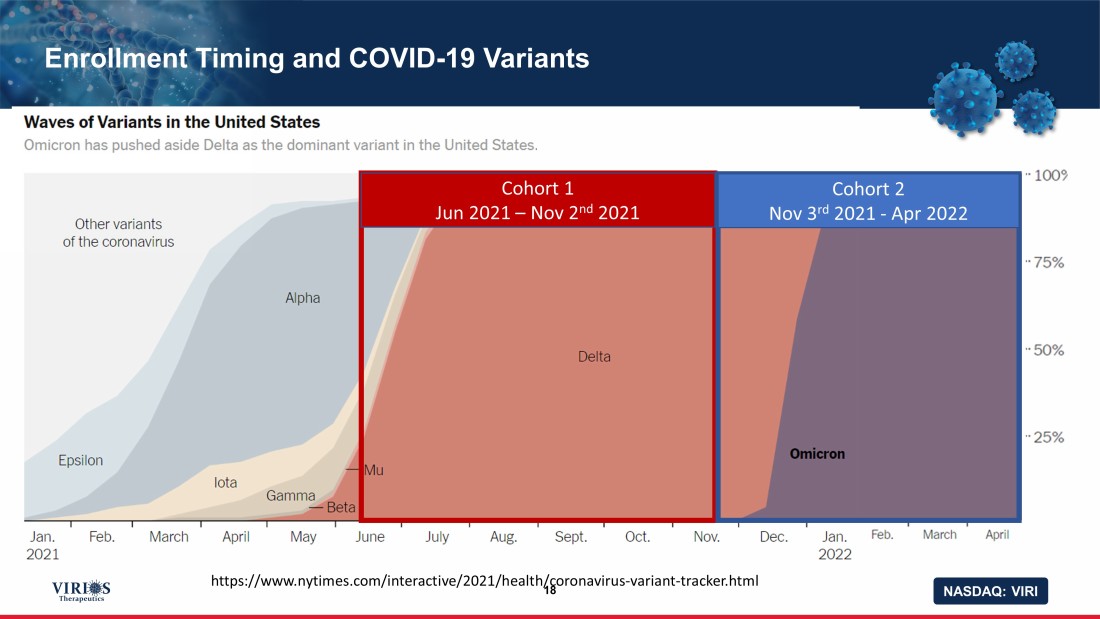
NASDAQ: VIRI Enrollment Timing and COVID - 19 Variants 18 https://www.nytimes.com/interactive/2021/health/coronavirus - variant - tracker.html Cohort 1 Jun 2021 – Nov 2 nd 2021 Cohort 2 Nov 3 rd 2021 - Apr 2022
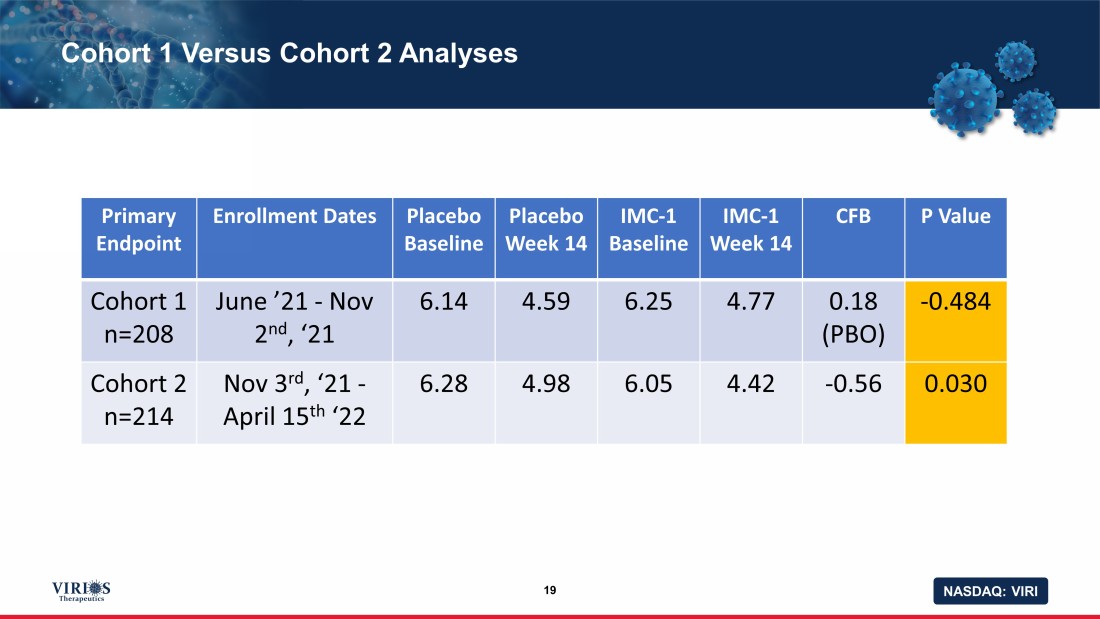
NASDAQ: VIRI Cohort 1 Versus Cohort 2 Analyses 19 Primary Endpoint Enrollment Dates Placebo Baseline Placebo Week 14 IMC - 1 Baseline IMC - 1 Week 14 CFB P Value Cohort 1 n=208 June ’21 - Nov 2 nd , ‘21 6.14 4.59 6.25 4.77 0.18 (PBO) - 0.484 Cohort 2 n=214 Nov 3 rd , ‘21 - April 15 th ‘22 6.28 4.98 6.05 4.42 - 0.56 0.030
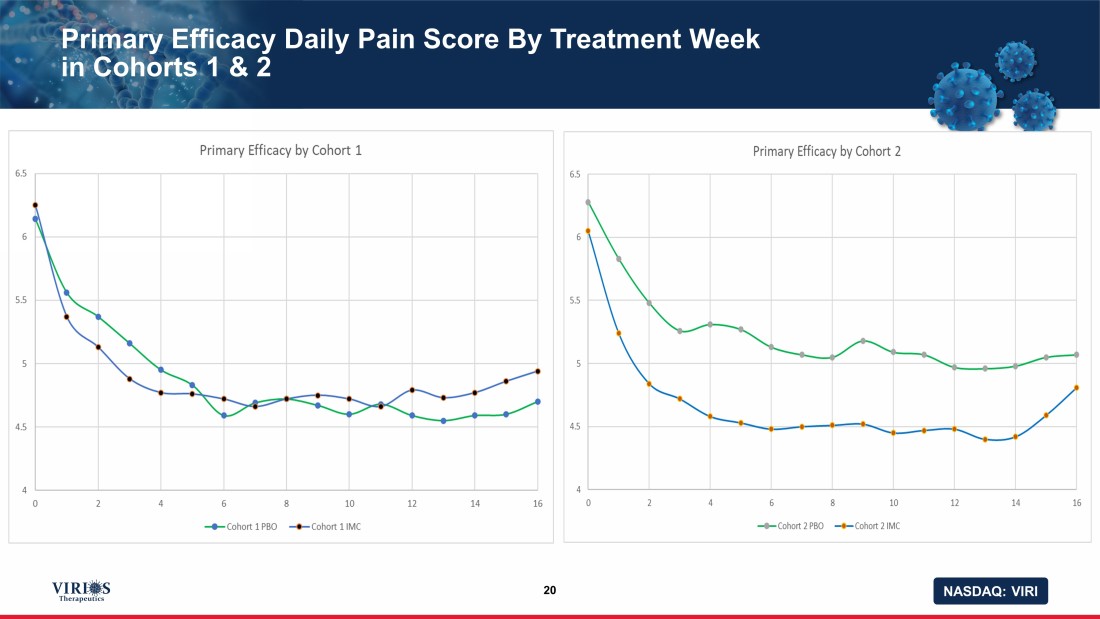
NASDAQ: VIRI Primary Efficacy Daily Pain Score By Treatment Week in Cohorts 1 & 2 20
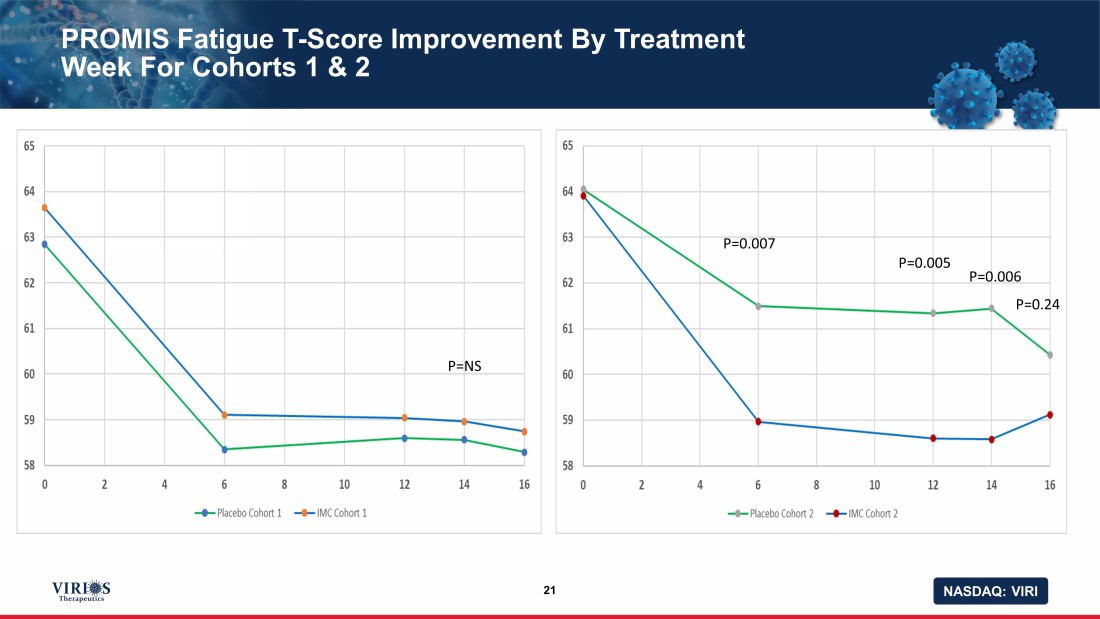
NASDAQ: VIRI PROMIS Fatigue T - Score Improvement By Treatment Week For Cohorts 1 & 2 21 P=NS P=0.007 P=0.005 P=0.006 P=0.24
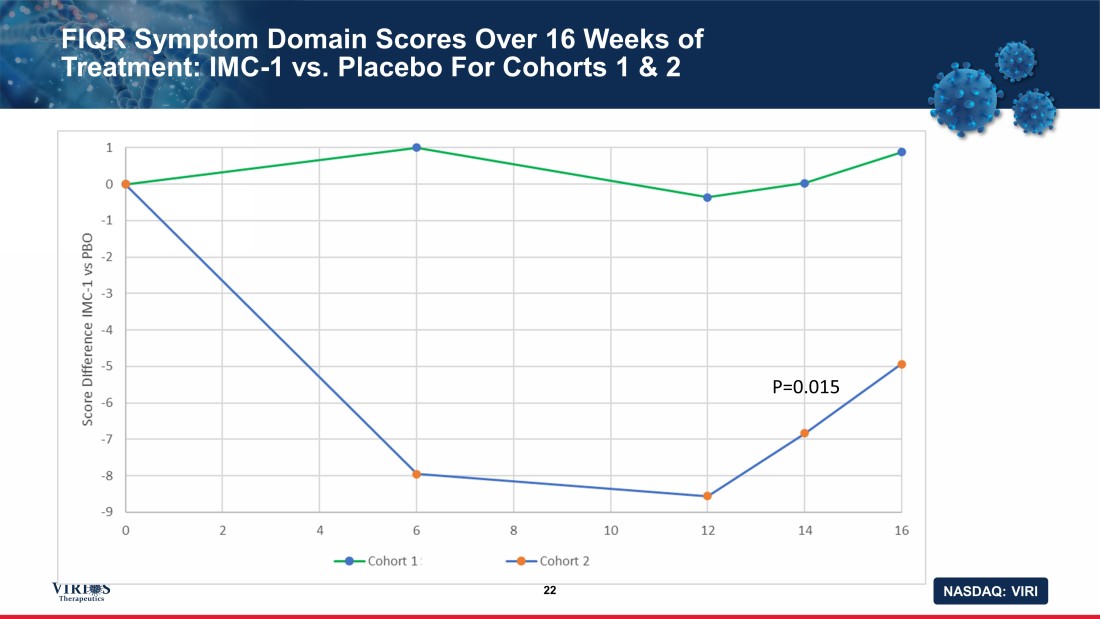
NASDAQ: VIRI FIQR Symptom Domain Scores Over 16 Weeks of Treatment: IMC - 1 vs. Placebo For Cohorts 1 & 2 22 P=0.015
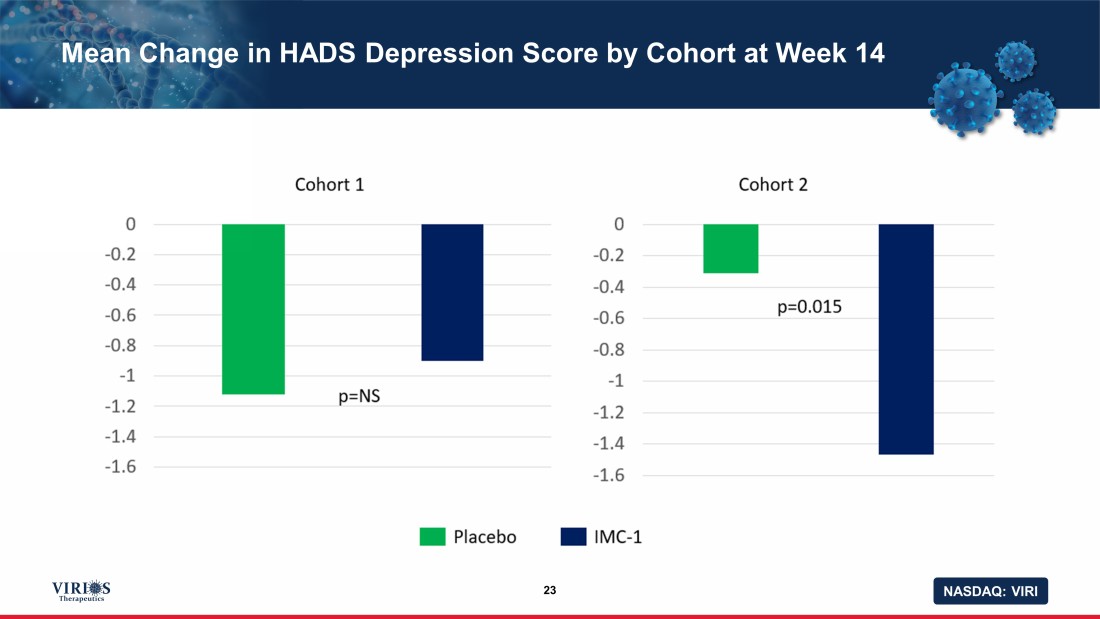
NASDAQ: VIRI Mean Change in HADS Depression Score by Cohort at Week 14 23

NASDAQ: VIRI FORTRESS Summary 24 □ Virios management strongly believes this mechanism has potential to improve FM patient care – Positive Phase 2a clinical study results – IMC - 1 in Cohort 2 delivered statistically significant improvement in FM patient pain, fatigue, depression and overall health status – IMC - 1 in Cohort 2 efficacy results were consistent with the expected profile from previous Phase 2a study – The difference in results between Cohort 1 and Cohort 2 is highly unlikely due to chance □ We believe the excellent overall safety and tolerability profile observed in FORTRESS supports future product development □ Our ultimate goal is to get IMC - 1 to market □ Our short - term plan is to engage with KOLs/ BoD to better understand the Phase 2b data and design a forward development plan to maximize the potential of IMC - 1
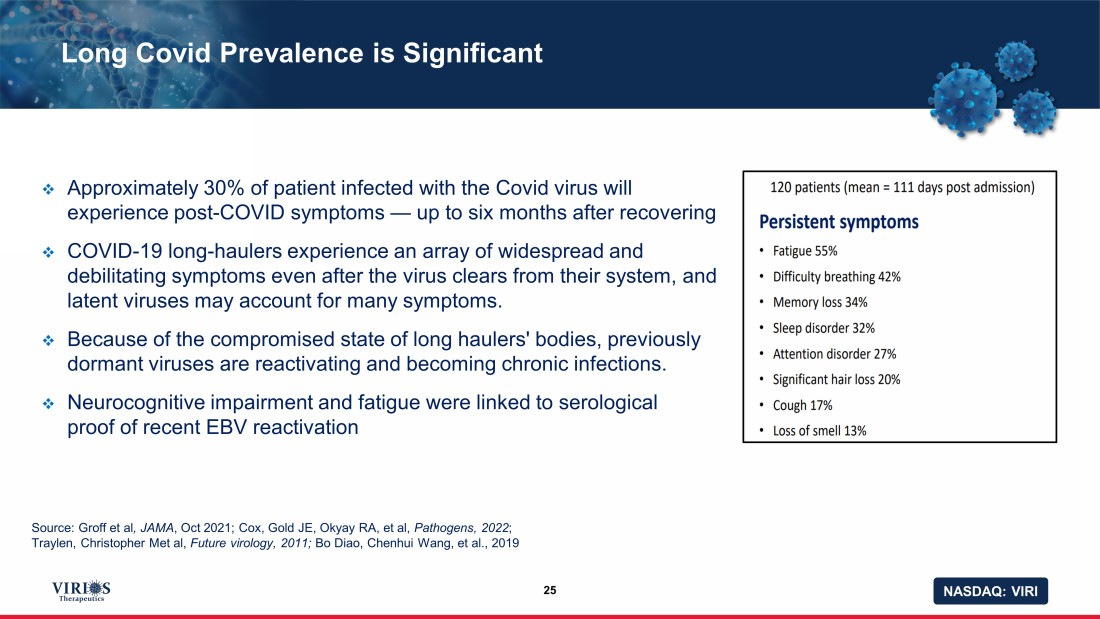
NASDAQ: VIRI Long Covid Prevalence is Significant 25 □ Approximately 30% of patient infected with the Covid virus will experience post - COVID symptoms — up to six months after recovering □ COVID - 19 long - haulers experience an array of widespread and debilitating symptoms even after the virus clears from their system, and latent viruses may account for many symptoms. □ Because of the compromised state of long haulers' bodies, previously dormant viruses are reactivating and becoming chronic infections. □ N eurocognitive impairment and fatigue were linked to serological proof of recent EBV reactivation Source: Groff et al , JAMA , Oct 2021; Cox, Gold JE, Okyay RA, et al, Pathogens, 2022 ; Traylen, Christopher Met al, Future virology, 2011; Bo Diao , Chenhui Wang, et al., 2019
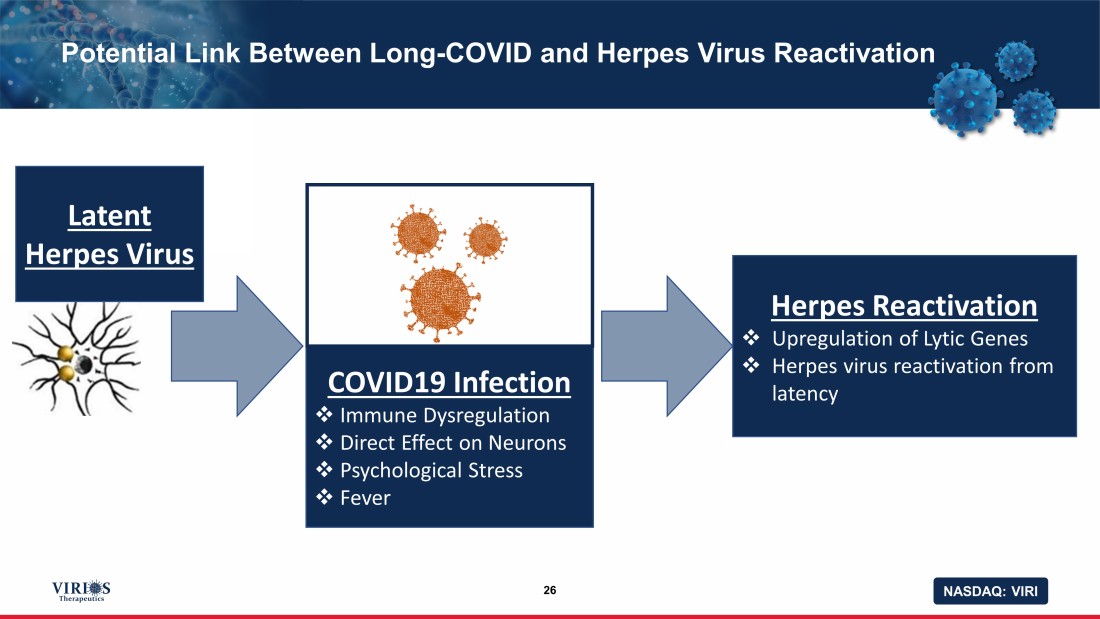
NASDAQ: VIRI COVID19 Infection □ Immune Dysregulation □ Direct Effect on Neurons □ Psychological Stress □ Fever Potential Link Between Long - COVID and Herpes Virus Reactivation 26 Herpes Reactivation □ Upregulation of Lytic Genes □ Herpes virus reactivation from latency Latent Herpes Virus

NASDAQ: VIRI Strong IP Portfolio with 21 Issued Patents to 2033 27 Four Issued US IMC - 1 P atents • Two “Composition of M a tter” P a tents • Drug - combin a tion of famcicl o vir and c e lec o xib • Synergistic combination for total daily dose of famciclovir and celecoxib • Two “Method - of - Use” P a tents • F amcicl o vir + c e lec o xib f or the t r e a tment of FM ( f ib r o m y a lgia), CFS or IBS • Method of dispensing famcicl o vir + c e lec o xib in a r egimen to t r e a t Function a l Som a tic Synd r ome conditions Six Issued F o r eign IMC - 1 P atents • Eu r opean P a tent validated in 18 countries • J a pan, A ust r a lia, China, South K o r ea and Canada Eight US P atents C ov ering Other Anti - V i r al Combinations • Various combinations of ac y cl o vir, m e l o xicam, diclofenac, famcicl o vir, v a lac y cl o vir, c e lec o xib
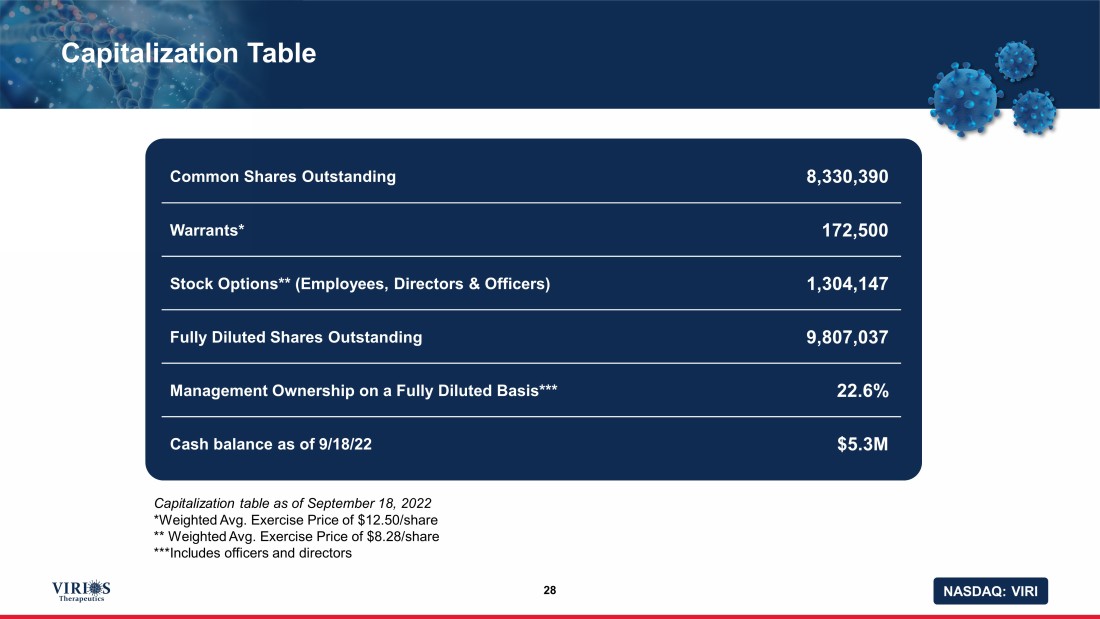
NASDAQ: VIRI Capitalization Table 28 Common Shares Outstanding 8,330,390 Warrants* 172,500 Stock Options** (Employees, Directors & Officers) 1,304,147 Fully Diluted Shares Outstanding 9,807,037 Management Ownership on a Fully Diluted Basis*** 22.6% Cash balance as of 9/18/22 $5.3M Capitalization table as of September 18, 2022 *Weighted Avg. Exercise Price of $12.50/share ** Weighted Avg. Exercise Price of $8.28/share ***Includes officers and directors

THANK YOU! www.virios.com For Additional Information, Contact: Kirin Smith : ksmith@pcgadvisory.com or ir@virios.com